Posttranslational Modification of Human Cytochrome CYP4F11 by 4-Hydroxynonenal Impairs ω-Hydroxylation in Malaria Pigment Hemozoin-Fed Monocytes: The Role in Malaria Immunosuppression
Abstract
:1. Introduction
2. Results
2.1. 4-HNE-Protein Conjugate Formation in Primary Human Monocytes Fed with HZ
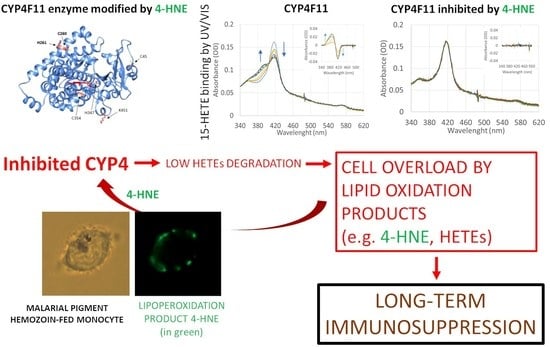
2.2. CYP4F11-4-HNE Conjugates in HZ-Fed Monocytes and Conjugation Sites in CYP4F11
2.3. In Vitro Production of Functional CYP4F11
2.4. Enzyme Modification by 4-HNE
2.5. Effect of CYP4F11 4-HNE Modification on Substrate Binding
2.6. Inhibition of Enzyme Activity of CYP4F11 by 4-HNE
2.7. Inhibition of ω-Hydroxylation Activity of CYP4F11 by 4-HNE Modification
2.8. Functional Impairment of Monocytes by 15-HETE: Inhibition of Oxidative Burst and Surface Antigen Expression in Monocyte Derived DC
3. Discussion
4. Materials and Methods
4.1. Culturing of Plasmodium Falciparum (Pf) and Isolation of HZ
4.2. Opsonisation of HZ and Latex Beads for Phagocytosis
4.3. Isolation of Monocytes, Phagocytosis of HZ and Latex Beads, and Treatment with 4-HNE
4.4. Differentiation of Dendritic Cells (DC) from Human Monocytes
4.5. Flow Cytometry Analysis of Cell Phenotype, Surface 4-HNE-Protein Conjugates and Apoptosis
4.6. Oxidative Burst Measurement
4.7. 4-HNE-Protein Conjugates Detection by Microscopy
4.8. 4-HNE-Protein Conjugates Detection by SDS-PAGE/Western Blotting (WB)
4.9. Identification of 4-HNE Binding Sites in CYP4F11 by Mass Spectrometry
4.10. CYP4F11 Expression and Purification
4.11. CO-Binding Spectral Assay
4.12. Recombinant CYP4F11 Modification by 4-HNE
4.13. Substrate Binding Assay
4.14. NADPH Consumption Assay
4.15. Gas Chromatography (GC) Analysis
4.16. Statistical Analysis
4.17. Data Sharing Statement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coronado, L.M.; Nadovich, C.T.; Spadafora, C. Malarial hemozoin: From target to tool. Biochim. Biophys. Acta 2014, 1840, 2032–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarzer, E.; Kuhn, H.; Valente, E.; Arese, P. Malaria-parasitized erythrocytes and hemozoin non-enzymatically generate large amounts of hydroxy fatty acids that inhibit monocyte functions. Blood 2003, 101, 722–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega-Pajares, A.; Rogerson, S.J. The Rough Guide to Monocytes in Malaria Infection. Front. Immunol. 2018, 9, 2888. [Google Scholar] [CrossRef]
- Metzger, W.G.; Mordmüller, B.G.; Kremsner, P.G. Malaria pigment in leucocytes. Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 637–638. [Google Scholar] [CrossRef]
- Lyke, K.E.; Diallo, D.A.; Dicko, A.; Kone, A.; Coulibaly, D.; Guindo, A.; Cissoko, Y.; Sangare, L.; Coulibaly, S.; Dakouo, B.; et al. Association of intraleukocytic Plasmodium falciparum malaria pigment with disease severity, clinical manifestations, and prognosis in severe malaria. Am. J. Trop. Med. Hyg. 2003, 69, 253–259. [Google Scholar] [CrossRef]
- Moiz, B.; Ali, S.S. Intraleucocytic haemozoin—A biomarker for severe malaria. Br. J. Haematol. 2016, 172, 313. [Google Scholar] [CrossRef] [Green Version]
- Schwarzer, E.; Turrini, F.; Ulliers, D.; Giribaldi, G.; Ginsburg, H.; Arese, P. Impairment of macrophage functions after ingestion of Plasmodium falciparum-infected erythrocytes or isolated malarial pigment. J. Exp. Med. 1992, 176, 1033–1041. [Google Scholar] [CrossRef] [Green Version]
- Gallo, V.; Skorokhod, O.A.; Schwarzer, E.; Arese, P. Simultaneous determination of phagocytosis of Plasmodium falciparum-parasitized and non-parasitized red blood cells by flow cytometry. Malar. J. 2012, 11, 428. [Google Scholar] [CrossRef] [Green Version]
- Skorokhod, O.A.; Barrera, V.; Heller, R.; Carta, F.; Turrini, F.; Arese, P.; Schwarzer, E. Malarial pigment hemozoin impairs chemotactic motility and transendothelial migration of monocytes via 4-hydroxynonenal. Free Radic. Biol. Med. 2014, 75, 210–221. [Google Scholar] [CrossRef]
- Schwarzer, E.; Skorokhod, O.A.; Barrera, V.; Arese, P. Hemozoin and the human monocyte—A brief review of their interactions. Parassitologia 2008, 50, 143–145. [Google Scholar] [PubMed]
- Schwarzer, E.; Arese, P.; Skorokhod, O.A. Role of the Lipoperoxidation product 4-hydroxynonenal in the pathogenesis of severe malaria anemia and malaria immunodepression. Oxidative Med. Cell. Longev. 2015, 2015, 638416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skorokhod, O.A.; Alessio, M.; Mordmüller, B.; Arese, P.; Schwarzer, E. Hemozoin (malarial pigment) inhibits differentiation and maturation of human monocyte-derived dendritic cells: A peroxisome proliferator-activated receptor-γ-mediated effect. J. Immunol. 2004, 173, 4066–4074. [Google Scholar] [CrossRef] [Green Version]
- Skorokhod, O.; Barrera, V.; Mandili, G.; Costanza, F.; Valente, E.; Ulliers, D.; Schwarzer, E. Malaria Pigment Hemozoin Impairs GM-CSF Receptor Expression and Function by 4-Hydroxynonenal. Antioxidants 2021, 10, 1259. [Google Scholar] [CrossRef] [PubMed]
- Barrera, V.; Skorokhod, O.A.; Baci, D.; Gremo, G.; Arese, P.; Schwarzer, E. Host fibrinogen stably bound to hemozoin rapidly activates monocytes via TLR-4 and CD11b/CD18-integrin: A new paradigm of hemozoin action. Blood 2011, 117, 5674–5682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, A.L.; Edson, K.Z.; Totah, R.A.; Rettie, A.E. Cytochrome P450 ω-Hydroxylases in Inflammation and Cancer. Adv. Pharmacol. 2015, 74, 223–262. [Google Scholar]
- Yi, M.; Shin, J.-G.; Lee, S.-J. Expression of CYP4V2 in human THP1 macrophages and its transcriptional regulation by peroxisome proliferator-activated receptor gamma. Toxicol. Appl. Pharmacol. 2017, 330, 100–106. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Kalsotra, A.; Turman, C.M.; Grill, R.J.; Dash, P.K.; Strobel, H.W. CYP4Fs expression in rat brain correlates with changes in LTB4 levels after traumatic brain injury. J. Neurotrauma 2008, 25, 1187–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehgal, N.; Agarwal, V.; Valli, R.K.; Joshi, S.D.; Antonovic, L.; Strobel, H.W.; Ravindranath, V. Cytochrome P4504f, a potential therapeutic target limiting neuroinflammation. Biochem. Pharmacol. 2011, 82, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Bell, J.C.; Keeney, D.S.; Strobel, H.W. Gene regulation of CYP4F11 in human keratinocyte HaCaT cells. Drug Metab. Dispos. 2010, 38, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edson, K.Z.; Rettie, A.E. CYP4 enzymes as potential drug targets: Focus on enzyme multiplicity, inducers and inhibitors, and therapeutic modulation of 20-hydroxyeicosatetraenoic acid (20-HETE) synthase and fatty acid ω-hydroxylase activities. Curr. Top. Med. Chem. 2013, 13, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Vaivoda, R.; Vaine, C.; Boerstler, C.; Galloway, K.; Christmas, P. CYP4F18-Deficient Neutrophils Exhibit Increased Chemotaxis to Complement Component C5a. J. Immunol. Res. 2015, 2015, 250456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theken, K.N.; Deng, Y.; Kannon, M.A.; Miller, T.M.; Poloyac, S.M.; Lee, C.R. Activation of the acute inflammatory response alters cytochrome P450 expression and eicosanoid metabolism. Drug Metab. Dispos. 2011, 39, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Poli, G.; Schaur, R.J. 4-Hydroxynonenal in the pathomechanism of oxidative stress. IUBMB Life 2000, 50, 315–321. [Google Scholar] [CrossRef]
- Schwarzer, E.; Müller, O.; Arese, P.; Siems, W.G.; Grune, T. Increased levels of 4-hydroxynonenal in human monocytes fed with malarial pigment hemozoin. A possible clue for hemozoin toxicity. FEBS Lett. 1996, 388, 119–122. [Google Scholar] [CrossRef] [Green Version]
- Bestervelt, L.L.; Vaz, A.D.; Coon, M.J. Inactivation of ethanol-inducible cytochrome P450 and other microsomal P450 isozymes by trans-4-hydroxy-2-nonenal, a major product of membrane lipid peroxidation. Proc. Natl. Acad. Sci. USA 1995, 92, 3764–3768. [Google Scholar] [CrossRef] [Green Version]
- Golizeh, M.; Geib, T.; Sleno, L. Identification of 4-hydroxynonenal protein targets in rat, mouse and human liver microsomes by two-dimensional liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2016, 30, 1488–1494. [Google Scholar] [CrossRef]
- Aldini, G.; Dalle-Donne, I.; Vistoli, G.; Maffei Facino, R.; Carini, M. Covalent modification of actin by 4-hydroxy-trans-2-nonenal (HNE): LC-ESI-MS/MS evidence for Cys374 Michael adduction. J. Mass Spectrom. 2005, 40, 946–954. [Google Scholar] [CrossRef]
- Kalsotra, A.; Turman, C.M.; Kikuta, Y.; Strobel, H.W. Expression and characterization of human cytochrome P450 4F11: Putative role in the metabolism of therapeutic drugs and eicosanoids. Toxicol. Appl. Pharmacol. 2004, 199, 295–304. [Google Scholar] [CrossRef]
- Skorokhod, O.; Schwarzer, E.; Grune, T.; Arese, P. Role of 4-hydroxynonenal in the hemozoin-mediated inhibition of differentiation of human monocytes to dendritic cells induced by GM-CSF/IL-4. Biofactors 2005, 24, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Urban, B.C.; Mwangi, T.; Ross, A.; Kinyanjui, S.; Mosobo, M.; Kai, O.; Lowe, B.; Marsh, K.; Roberts, D.J. Peripheral blood dendritic cells in children with acute Plasmodium falciparum malaria. Blood 2001, 98, 2859–2861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calle, C.L.; Mordmüller, B.; Singh, A. Immunosuppression in Malaria: Do Plasmodium falciparum Parasites Hijack the Host? Pathogens 2021, 10, 1277. [Google Scholar] [CrossRef]
- Dostert, C.; Guarda, G.; Romero, J.F.; Menu, P.; Gross, O.; Tardivel, A.; Suva, M.-L.; Stehle, J.-C.; Kopf, M.; Stamenkovic, I.; et al. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS ONE 2009, 4, e6510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyler, D.J. Cellular aspects of immunoregulation in malaria. Bull. World Health Organ. 1979, 57, 239–243. [Google Scholar] [PubMed]
- Harding, C.L.; Villarino, N.F.; Valente, E.; Schwarzer, E.; Schmidt, N.W. Plasmodium Impairs Antibacterial Innate Immunity to Systemic Infections in Part Through Hemozoin-Bound Bioactive Molecules. Front. Cell. Infect. Microbiol. 2020, 10, 328. [Google Scholar] [CrossRef] [PubMed]
- Fiori, P.L.; Rappelli, P.; Mirkarimi, S.N.; Ginsburg, H.; Cappuccinelli, P.; Turrini, F. Reduced microbicidal and anti-tumour activities of human monocytes after ingestion of Plasmodium falciparum-infected red blood cells. Parasite Immunol. 1993, 15, 647–655. [Google Scholar] [CrossRef]
- Bujila, I.; Schwarzer, E.; Skorokhod, O.; Weidner, J.M.; Troye-Blomberg, M.; Farrants, A.-K.Ö. Malaria-derived hemozoin exerts early modulatory effects on the phenotype and maturation of human dendritic cells. Cell. Microbiol. 2016, 18, 413–423. [Google Scholar] [CrossRef] [Green Version]
- Schwarzer, E.; Ludwig, P.; Valente, E.; Arese, P. 15(S)-hydroxyeicosatetraenoic acid (15-HETE), a product of arachidonic acid peroxidation, is an active component of hemozoin toxicity to monocytes. Parassitologia 1999, 41, 199–202. [Google Scholar]
- Urban, B.C.; Todryk, S. Malaria pigment paralyzes dendritic cells. J. Biol. 2006, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Schwarzer, E.; Bellomo, G.; Giribaldi, G.; Ulliers, D.; Arese, P. Phagocytosis of malarial pigment haemozoin by human monocytes: A confocal microscopy study. Parasitology 2001, 123, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Salamanca-Pinzón, S.G.; Wu, Z.-L.; Xiao, Y.; Guengerich, F.P. Human cytochrome P450 4F11: Heterologous expression in bacteria, purification, and characterization of catalytic function. Arch. Biochem. Biophys. 2010, 494, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, Y.; Kodai, S.; Takemura, S.; Minamiyama, Y.; Niki, E. Simultaneous measurement of F2-isoprostane, hydroxyoctadecadienoic acid, hydroxyeicosatetraenoic acid, and hydroxycholesterols from physiological samples. Anal. Biochem. 2008, 379, 105–115. [Google Scholar] [CrossRef]
- Skorokhod, O.A.; Caione, L.; Marrocco, T.; Migliardi, G.; Barrera, V.; Arese, P.; Piacibello, W.; Schwarzer, E. Inhibition of erythropoiesis in malaria anemia: Role of hemozoin and hemozoin-generated 4-hydroxynonenal. Blood 2010, 116, 4328–4337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skorokhod, O.A.; Davalos-Schafler, D.; Gallo, V.; Valente, E.; Ulliers, D.; Notarpietro, A.; Mandili, G.; Novelli, F.; Persico, M.; Taglialatela-Scafati, O.; et al. Oxidative stress-mediated antimalarial activity of plakortin, a natural endoperoxide from the tropical sponge Plakortis simplex. Free Radic. Biol. Med. 2015, 89, 624–637. [Google Scholar] [CrossRef]
- Hausjell, J.; Halbwirth, H.; Spadiut, O. Recombinant production of eukaryotic cytochrome P450s in microbial cell factories. Biosci. Rep. 2018, 38, BSR20171290. [Google Scholar] [CrossRef] [Green Version]
- Omura, T.; Sato, R. The Carbon Monoxide-Binding Pigment of Liver Microsomes. I. Evidence for Its Hemoprotein Nature. J. Biol. Chem. 1964, 239, 2370–2378. [Google Scholar] [CrossRef]









| Substrate | Dissociation Constants Kd (µM) |
|---|---|
| Palmitic acid (PA) | 52.1 ± 4.0 |
| Arachidonic acid (AA) | 97.8 ± 16.2 |
| 12-HETE | 38.2 ± 2.5 |
| 15-HETE | 73.3 ± 13.5 |
| Substrate | Control Experiment | CYP4F11 | 4-HNE-Conjugated CYP4F11 |
|---|---|---|---|
| Palmitic acid (PA) | 3.0 ± 0.8 | 6.2 ± 1.4 *,§ | 3.5 ± 0.7 |
| Arachidonic acid (AA) | 2.1 ± 0.7 | 6.1 ± 2.2 * | 3.1 ± 0.9 |
| 12-HETE | 2.6 ± 1.2 | 22.3 ± 2.5 *,§ | 3.7 ± 1.3 |
| 15-HETE | 2.4 ± 1.0 | 33.4 ± 8.1 *,§ | 4.3 ± 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skorokhod, O.; Triglione, V.; Barrera, V.; Di Nardo, G.; Valente, E.; Ulliers, D.; Schwarzer, E.; Gilardi, G. Posttranslational Modification of Human Cytochrome CYP4F11 by 4-Hydroxynonenal Impairs ω-Hydroxylation in Malaria Pigment Hemozoin-Fed Monocytes: The Role in Malaria Immunosuppression. Int. J. Mol. Sci. 2023, 24, 10232. https://doi.org/10.3390/ijms241210232
Skorokhod O, Triglione V, Barrera V, Di Nardo G, Valente E, Ulliers D, Schwarzer E, Gilardi G. Posttranslational Modification of Human Cytochrome CYP4F11 by 4-Hydroxynonenal Impairs ω-Hydroxylation in Malaria Pigment Hemozoin-Fed Monocytes: The Role in Malaria Immunosuppression. International Journal of Molecular Sciences. 2023; 24(12):10232. https://doi.org/10.3390/ijms241210232
Chicago/Turabian StyleSkorokhod, Oleksii, Vincenzo Triglione, Valentina Barrera, Giovanna Di Nardo, Elena Valente, Daniela Ulliers, Evelin Schwarzer, and Gianfranco Gilardi. 2023. "Posttranslational Modification of Human Cytochrome CYP4F11 by 4-Hydroxynonenal Impairs ω-Hydroxylation in Malaria Pigment Hemozoin-Fed Monocytes: The Role in Malaria Immunosuppression" International Journal of Molecular Sciences 24, no. 12: 10232. https://doi.org/10.3390/ijms241210232
APA StyleSkorokhod, O., Triglione, V., Barrera, V., Di Nardo, G., Valente, E., Ulliers, D., Schwarzer, E., & Gilardi, G. (2023). Posttranslational Modification of Human Cytochrome CYP4F11 by 4-Hydroxynonenal Impairs ω-Hydroxylation in Malaria Pigment Hemozoin-Fed Monocytes: The Role in Malaria Immunosuppression. International Journal of Molecular Sciences, 24(12), 10232. https://doi.org/10.3390/ijms241210232