Fluorinated Benzofuran and Dihydrobenzofuran as Anti-Inflammatory and Potential Anticancer Agents
Abstract
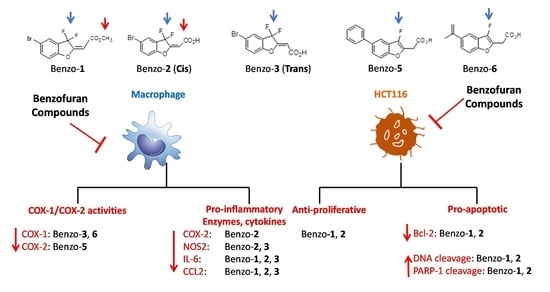
:1. Introduction
2. Results and Discussion
2.1. Effect of Inflammation on Macrophages
2.2. Effects of Compounds 2, 3, 5, and 6 on Inflammation in Subcutaneous Zymosan-Induced Air Pouch in Mice
2.3. Anticancer Effect
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Fluorinated Benzofuran
3.3. Evaluation of Inflammation in Bone Marrow-Derived Macrophages (BMDMs)
3.4. Cell Treatment
3.5. Cyclooxygenase Activity
3.6. Subcutaneous Dorsal Air Pouch Model
3.7. Reverse Transcriptase-PCR (RT-PCR)
3.8. Toxicity Assay
3.9. Proliferation Assay
3.10. Western Blot
3.11. TUNEL Assay
3.12. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rankin, L.C.; Artis, D. Beyond Host Defense: Emerging Functions of the Immune System in Regulating Complex Tissue Physiology. Cell 2018, 173, 554–567. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korniluk, A.; Koper, O.; Kemona, H.; Dymicka-Piekarska, V. From inflammation to cancer. Ir. J. Med. Sci. 2017, 186, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izano, M.; Wei, E.K.; Tai, C.; Swede, H.; Gregorich, S.; Harris, T.B.; Klepin, H.; Satterfield, S.; Murphy, R.; Newman, A.B.; et al. Chronic inflammation and risk of colorectal and other obesity-related cancers: The health, aging and body composition study. Int. J. Cancer 2016, 138, 1118–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamoya, T.; Fujii, G.; Miyamoto, S.; Takahashi, M.; Totsuka, Y.; Wakabayashi, K.; Toshima, J.; Mutoh, M. Effects of NSAIDs on the risk factors of colorectal cancer: A mini review. Genes Environ. 2016, 38, 6. [Google Scholar] [CrossRef] [Green Version]
- Patrignani, P.; Patrono, C. Aspirin and Cancer. J. Am. Coll. Cardiol. 2016, 68, 967–976. [Google Scholar] [CrossRef]
- Wang, D.; Dubois, R.N. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 2010, 29, 781–788. [Google Scholar] [CrossRef] [Green Version]
- Greenhough, A.; Smartt, H.J.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX-2/PGE2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009, 30, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Vainer, N.; Dehlendorff, C.; Johansen, J.S. Systematic literature review of IL-6 as a biomarker or treatment target in patients with gastric, bile duct, pancreatic and colorectal cancer. Oncotarget 2018, 9, 29820–29841. [Google Scholar] [CrossRef] [Green Version]
- Liao, W.; Ye, T.; Liu, H. Prognostic Value of Inducible Nitric Oxide Synthase (iNOS) in Human Cancer: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2019, 2019, 6304851. [Google Scholar] [CrossRef] [Green Version]
- Napiorkowska, M.; Cieslak, M.; Kazmierczak-Baranska, J.; Krolewska-Golinska, K.; Nawrot, B. Synthesis of New Derivatives of Benzofuran as Potential Anticancer Agents. Molecules 2019, 24, 1529. [Google Scholar] [CrossRef] [Green Version]
- Kao, C.; Chen, J. A convenient synthesis of naturally occurring benzofuran ailanthoidol. Tetrahedron 2001, 42, 1111–1113. [Google Scholar] [CrossRef]
- Liu, Z.; Xia, G.; Chen, S.; Liu, Y.; Li, H.; She, Z. Eurothiocin A and B, sulfur-containing benzofurans from a soft coral-derived fungus Eurotium rubrum SH-823. Mar. Drugs 2014, 12, 3669–3680. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Rathinasamy, K.; Mohan, R.; Panda, D. Microtubule assembly dynamics: An attractive target for anticancer drugs. IUBMB Life 2008, 60, 368–375. [Google Scholar] [CrossRef]
- Ho, Y.S.; Duh, J.S.; Jeng, J.H.; Wang, Y.J.; Liang, Y.C.; Lin, C.H.; Tseng, C.J.; Yu, C.F.; Chen, R.J.; Lin, J.K. Griseofulvin potentiates antitumorigenesis effects of nocodazole through induction of apoptosis and G2/M cell cycle arrest in human colorectal cancer cells. Int. J. Cancer 2001, 91, 393–401. [Google Scholar] [CrossRef]
- Costa-Pereira, A.P. Regulation of IL-6-type cytokine responses by MAPKs. Biochem. Soc. Trans. 2014, 42, 59–62. [Google Scholar] [CrossRef]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Hirz, T.; Khalaf, A.; El-Hachem, N.; Mrad, M.F.; Abdallah, H.; Creminon, C.; Gree, R.; Merhi, R.A.; Habib, A.; Hachem, A.; et al. New analogues of 13-hydroxyocatdecadienoic acid and 12-hydroxyeicosatetraenoic acid block human blood platelet aggregation and cyclooxygenase-1 activity. Chem. Central J. 2012, 6, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Achkar, G.A.; Jouni, M.; Mrad, M.F.; Hirz, T.; El Hachem, N.; Khalaf, A.; Hammoud, S.; Fayyad-Kazan, H.; Eid, A.A.; Badran, B.; et al. Thiazole derivatives as inhibitors of cyclooxygenases in vitro and in vivo. Eur. J. Pharmacol. 2015, 750, 66–73. [Google Scholar] [CrossRef] [PubMed]
- El-Achkar, G.A.; Mrad, M.F.; Mouawad, C.A.; Badran, B.; Jaffa, A.A.; Motterlini, R.; Hamade, E.; Habib, A. Heme oxygenase-1-Dependent anti-inflammatory effects of atorvastatin in zymosan-injected subcutaneous air pouch in mice. PLoS ONE 2019, 14, e0216405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posadas, I.; Terencio, M.C.; Guillen, I.; Ferrandiz, M.L.; Coloma, J.; Paya, M.; Alcaraz, M.J. Co-regulation between cyclo-oxygenase-2 and inducible nitric oxide synthase expression in the time-course of murine inflammation. Naunyn Schmiedebergs Arch. Pharmacol. 2000, 361, 98–106. [Google Scholar] [CrossRef]
- Vicente, A.M.; Guillen, M.I.; Habib, A.; Alcaraz, M.J. Beneficial effects of heme oxygenase-1 up-regulation in the development of experimental inflammation induced by zymosan. J. Pharmacol. Exp. Ther. 2003, 307, 1030–1037. [Google Scholar] [CrossRef]
- Plesca, D.; Mazumder, S.; Almasan, A. DNA damage response and apoptosis. Methods Enzymol. 2008, 446, 107–122. [Google Scholar] [CrossRef] [Green Version]
- Edlich, F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem. Biophys. Res. Commun. 2018, 500, 26–34. [Google Scholar] [CrossRef]
- Chaitanya, G.V.; Steven, A.J.; Babu, P.P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. 2010, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Hariss, L.; Bouhadir, K.; Roisnel, T.; Grée, R.; Hachem, A. Synthesis of Novel Fluorinated Benzofurans and Dihydrobenzofurans. Synlett 2017, 28, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Habib, A.; Chokr, D.; Wan, J.; Hegde, P.; Mabire, M.; Siebert, M.; Ribeiro-Parenti, L.; Le Gall, M.; Lettéron, P.; Pilard, N.; et al. Inhibition of monoacylglycerol lipase, an anti-inflammatory and antifibrogenic strategy in the liver. Gut 2018, 68, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, A.J.; Hariss, L.; El-Hachem, N.; El-Achkar, G.A.; Ghayad, S.E.; Dagher, O.K.; Borghol, N.; Gree, R.; Badran, B.; Hachem, A.; et al. gem-Difluorobisarylic derivatives: Design, synthesis and anti-inflammatory effect. BMC Chem. 2019, 13, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodder, J.; Denaes, T.; Chobert, M.N.; Wan, J.; El-Benna, J.; Pawlotsky, J.M.; Lotersztajn, S.; Teixeira-Clerc, F. Macrophage autophagy protects against liver fibrosis in mice. Autophagy 2015, 11, 1280–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, J.; Benkdane, M.; Teixeira-Clerc, F.; Bonnafous, S.; Louvet, A.; Lafdil, F.; Pecker, F.; Tran, A.; Gual, P.; Mallat, A.; et al. M2 Kupffer cells promote M1 Kupffer cell apoptosis: A protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology 2014, 59, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.; Rautou, P.E.; Fasseu, M.; Giabicani, M.; de Chambrun, M.; Wan, J.; Minsart, C.; Gustot, T.; Couvineau, A.; Maiwall, R.; et al. Type I interferon signaling in systemic immune cells from patients with alcoholic cirrhosis and its association with outcome. J. Hepatol. 2017, 66, 930–941. [Google Scholar] [CrossRef]
- Habib, A.; Creminon, C.; Frobert, Y.; Grassi, J.; Pradelles, P.; Maclouf, J. Demonstration of an inducible cyclooxygenase in human endothelial cells using antibodies raised against the carboxyl-terminal region of the cyclooxygenase-2. J. Biol. Chem. 1993, 268, 23448–23454. [Google Scholar] [CrossRef] [PubMed]
- Eligini, S.; Habib, A.; Lebret, M.; Creminon, C.; Levy-Toledano, S.; Maclouf, J. Induction of cyclo-oxygenase-2 in human endothelial cells by SIN-1 in the absence of prostaglandin production. Br. J. Pharmacol. 2001, 133, 1163–1171. [Google Scholar] [CrossRef] [Green Version]
- Khanam, H.; Shamsuzzaman. Bioactive Benzofuran derivatives: A review. Eur. J. Med. Chem. 2015, 97, 483–504. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Westwell, A.D. The role of fluorine in medicinal chemistry. J. Enzym. Inhib. Med. Chem. 2007, 22, 527–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.Y.; Yuzhalin, A.E.; Gordon-Weeks, A.N.; Muschel, R.J. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget 2016, 7, 28697–28710. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.; Selvarajan, K.; Hasan, M.R.; Chan, A.P.; Jin, C.; Kim, J.; Chan, S.K.; Le, N.D.; Kim, Y.B.; Tai, I.T. Inhibition of COX-2 in colon cancer modulates tumor growth and MDR-1 expression to enhance tumor regression in therapy-refractory cancers in vivo. Neoplasia 2012, 14, 624–633. [Google Scholar] [CrossRef] [Green Version]
- Sobolewski, C.; Cerella, C.; Dicato, M.; Ghibelli, L.; Diederich, M. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int. J. Cell Biol. 2010, 2010, 215158. [Google Scholar] [CrossRef] [Green Version]






| Compound | IC50 (µM) | |||
|---|---|---|---|---|
| PGE2 | IL-6 | CCL2 | NO | |
| 1 | ND | ND | 8 | ND |
| 2 | 1.91 | 1.23 | 1.52 | 2.42 |
| 3 | 1.48 | 5.21 | 1.5 | 5.23 |
| 5 | 1.92 | ND | ND | ND |
| 6 | 1.12 | ND | ND | ND |
| 8 | 20.52 | 9.04 | 19.27 | ND |
| Compound | Starting Compound | Yield |
|---|---|---|
| 2 |  | 63% |
| 3 |  | 70% |
| 4 |  | 78% |
| 5 |  | 68% |
| 6 |  | 71% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayoub, A.J.; El-Achkar, G.A.; Ghayad, S.E.; Hariss, L.; Haidar, R.H.; Antar, L.M.; Mallah, Z.I.; Badran, B.; Grée, R.; Hachem, A.; et al. Fluorinated Benzofuran and Dihydrobenzofuran as Anti-Inflammatory and Potential Anticancer Agents. Int. J. Mol. Sci. 2023, 24, 10399. https://doi.org/10.3390/ijms241210399
Ayoub AJ, El-Achkar GA, Ghayad SE, Hariss L, Haidar RH, Antar LM, Mallah ZI, Badran B, Grée R, Hachem A, et al. Fluorinated Benzofuran and Dihydrobenzofuran as Anti-Inflammatory and Potential Anticancer Agents. International Journal of Molecular Sciences. 2023; 24(12):10399. https://doi.org/10.3390/ijms241210399
Chicago/Turabian StyleAyoub, Abeer J., Ghewa A. El-Achkar, Sandra E. Ghayad, Layal Hariss, Razan H. Haidar, Leen M. Antar, Zahraa I. Mallah, Bassam Badran, René Grée, Ali Hachem, and et al. 2023. "Fluorinated Benzofuran and Dihydrobenzofuran as Anti-Inflammatory and Potential Anticancer Agents" International Journal of Molecular Sciences 24, no. 12: 10399. https://doi.org/10.3390/ijms241210399
APA StyleAyoub, A. J., El-Achkar, G. A., Ghayad, S. E., Hariss, L., Haidar, R. H., Antar, L. M., Mallah, Z. I., Badran, B., Grée, R., Hachem, A., Hamade, E., & Habib, A. (2023). Fluorinated Benzofuran and Dihydrobenzofuran as Anti-Inflammatory and Potential Anticancer Agents. International Journal of Molecular Sciences, 24(12), 10399. https://doi.org/10.3390/ijms241210399