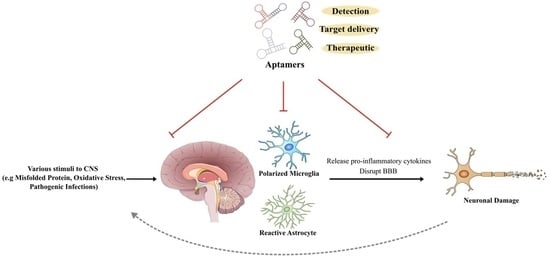
Exploring the Potential of Aptamers in Targeting Neuroinflammation and Neurodegenerative Disorders: Opportunities and Challenges
Abstract
:1. Introduction
2. Neuroinflammation
2.1. Role of Microglia
2.2. Activation of Microglia
2.3. Morphological Plasticity
2.4. Persistent Neuroinflammation
2.5. Therapeutic Applications of Immunomodulatory Medications in NDDs
| Classification | Feature | Example | References |
|---|---|---|---|
| 1. NSAIDs |
| Aspirin, Celecoxib, Naproxen, Mefenamic acid | [70] |
| 2. Immunomodulatory drugs | |||
| a. Monoclonal Antibodies |
| Natalizumab, Rituximab, Ofatumumab | [76,79] |
| b. Biologic drugs (e.g., TNF-α inhibitor) |
| Etanercept | [80] |
| 3. Phytochemical compounds |
| Resveratrol, Curcumin, Quercetin | [84,101] |
| 4. Others | |||
| Tetracycline Antibiotics |
| Minocycline, Doxycycline | [86,99] |
3. Aptamers
3.1. SELEX
3.2. Modifications of Aptamers
3.3. Limitations of Aptamers
4. Aptamer-Based Targeted Brain Delivery
4.1. Route of Administration
4.2. Can Aptamers Penetrate the Blood–Brain Barrier?
4.3. Aptamers Encapsulated by Exosomes to Bypass the Blood–Brain Barrier
4.4. Aptamers Targeting the Membrane Transferrin Receptor (TfR)
5. Diagnostic & Therapeutic Aptamers for Inflammatory Biomolecules in NDDs
5.1. Aptamers for the Detection of Neuroinflammatory Biomarkers
5.2. Therapeutic Aptamers Targeting Hallmark Proteins in NDDs
5.2.1. Aptamers against Aβ and BACE1 in AD
5.2.2. Aptamers against α-Syn in PD
6. Therapeutic Aptamers Targeting Neuroinflammation
6.1. Aptamers Targeting Activated Microglia & Damaged Neurons
6.2. Aptamers Targeting Proinflammatory Cytokines and Chemokines
6.3. Aptamers Targeting Cell Surface Receptors
6.4. Aptamers Targeting the Complement System & Membrane Components
7. Emerging Opportunities and Complex Challenges of Aptamers in NDDs
7.1. Emerging Opportunities
7.2. Complex Challenges
8. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| ADME | Absorption, Distribution, Metabolism, and Excretion |
| AML | Acute Myeloid Leukaemia |
| APOE | Apolipoprotein E |
| APP | Amyloid Precursor Protein |
| BACE | Beta-Secretase |
| BBB | Blood–Brain Barrier |
| BMSC | Bone Marrow-Derived Mesenchymal Stem Cells |
| CD | Cluster of Differentiation |
| CME | Clathrin-Mediated Endocytosis |
| CNS | Central Nervous System |
| DAM | Disease-Associated Microglia |
| DAMP | Damage-Associated Molecular Pattern |
| DOX | Doxorubicin |
| ECM | Extracellular Matrix |
| EpCAM | Epithelial Cell Adhesion Molecule |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-Activated Protein Kinase |
| MRCC | Metastatic Renal Cell Carcinoma |
| MS | Multiple Sclerosis |
| MST1 | Macrophage Stimulating kinase 1 |
| MYD88 | Myeloid Differentiation Primary Response 88 |
| NDDs | Neurodegenerative Diseases |
| NSAIDs | Non-Steroidal Anti-Inflammatory Drugs |
| PAMP | Pathogen-Associated Molecular Pattern |
| PD | Parkinson’s Disease |
| PEG | Polyethylene Glycol |
| PML | Progressive Multifocal Leukoencephalopathy |
| POX | Paraoxon |
| PPAR | Peroxisome Proliferator-Activated Receptor |
| PRRs | Pattern Recognition Receptors |
| PS | Phosphatidylserine |
| ROS | Reactive Oxygen Species |
| RVG | Rabies Virus Glycoprotein |
| SELEX | Systematic Evolution of Ligands by Exponential Enrichment |
| TBI | Traumatic Brain Injury |
| TLR | Toll-Like Receptor |
| TREM | Triggering Receptor Expressed on Myeloid Cells |
References
- Liang, Z.; Li, X.; Luo, X.; Luo, H.; Chen, Y.; Cai, M.; Zhong, X.; Fang, Y.; Guo, T.; Shi, Y.; et al. The Aptamer Ob2, a novel AChE inhibitor, restores cognitive deficits and alleviates amyloidogenesis in 5×FAD transgenic mice. Mol. Ther. Nucleic Acids 2022, 28, 114–123. [Google Scholar] [CrossRef]
- Kutovyi, Y.; Hlukhova, H.; Boichuk, N.; Menger, M.; Offenhäusser, A.; Vitusevich, S. Amyloid-beta peptide detection via aptamer-functionalized nanowire sensors exploiting single-trap phenomena. Biosens. Bioelectron. 2020, 154, 112053. [Google Scholar] [CrossRef] [PubMed]
- Michalska, P.; León, R. When It Comes to an End: Oxidative Stress Crosstalk with Protein Aggregation and Neuroinflammation Induce Neurodegeneration. Antioxidants 2020, 9, 740. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.I.P.; Staniforth, R.A. General Principles Underpinning Amyloid Structure. Front. Neurosci. 2022, 16, 878869. [Google Scholar] [CrossRef] [PubMed]
- Jagust, W. Is amyloid-β harmful to the brain? Insights from human imaging studies. Brain 2016, 139 Pt 1, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Knopman, D.S.; Jack, C.R., Jr.; Wiste, H.J.; Weigand, S.D.; Vemuri, P.; Lowe, V.J.; Kantarci, K.; Gunter, J.L.; Senjem, M.L.; Mielke, M.M.; et al. Brain injury biomarkers are not dependent on β-amyloid in normal elderly. Ann. Neurol. 2013, 73, 472–480. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Lu, L.; Pember, E.; Li, X.; Zhang, B.; Zhu, Z. New Insights into Neuroinflammation Involved in Pathogenic Mechanism of Alzheimer’s Disease and Its Potential for Therapeutic Intervention. Cells 2022, 11, 1925. [Google Scholar] [CrossRef]
- Dijkstra, A.A.; Voorn, P.; Berendse, H.W.; Groenewegen, H.J.; Rozemuller, A.J.; van de Berg, W.D. Stage-dependent nigral neuronal loss in incidental Lewy body and Parkinson’s disease. Mov. Disord. 2014, 29, 1244–1251. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Obeso, J.A.; Halliday, G.M. Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.M.; Yang, D.; Li, X.Q.; Liu, J.; Back, T.C.; Trivett, A.; Karim, B.; Barbut, D.; Zasloff, M.; Oppenheim, J.J. Alpha synuclein, the culprit in Parkinson disease, is required for normal immune function. Cell Rep. 2022, 38, 110090. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef]
- Chu, C.; Artis, D.; Chiu, I.M. Neuro-immune Interactions in the Tissues. Immunity 2020, 52, 464–474. [Google Scholar] [CrossRef]
- Tan, S.H.; Karri, V.; Tay, N.W.R.; Chang, K.H.; Ah, H.Y.; Ng, P.Q.; Ho, H.S.; Keh, H.W.; Candasamy, M. Emerging pathways to neurodegeneration: Dissecting the critical molecular mechanisms in Alzheimer’s disease, Parkinson’s disease. Biomed. Pharmacother. 2019, 111, 765–777. [Google Scholar] [CrossRef]
- Amor, S.; Puentes, F.; Baker, D.; van der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Gilhus, N.E.; Deuschl, G. Neuroinflammation—A common thread in neurological disorders. Nat. Rev. Neurol. 2019, 15, 429–430. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. S2), 136–153. [Google Scholar] [CrossRef] [Green Version]
- Konsman, J.P. Cytokines in the Brain and Neuroinflammation: We Didn’t Starve the Fire! Pharmaceuticals 2022, 15, 140. [Google Scholar] [CrossRef]
- Yong, H.Y.F.; Rawji, K.S.; Ghorbani, S.; Xue, M.; Yong, V.W. The benefits of neuroinflammation for the repair of the injured central nervous system. Cell Mol. Immunol. 2019, 16, 540–546. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Chakraborty, B.; Mukerjee, N.; Maitra, S.; Zehravi, M.; Mukherjee, D.; Ghosh, A.; Massoud, E.E.S.; Rahman, M.H. Therapeutic Potential of Different Natural Products for the Treatment of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2022, 2022, 6873874. [Google Scholar] [CrossRef]
- Karri, V.; Ramos, D.; Martinez, J.B.; Odena, A.; Oliveira, E.; Coort, S.L.; Evelo, C.T.; Mariman, E.C.M.; Schuhmacher, M.; Kumar, V. Differential protein expression of hippocampal cells associated with heavy metals (Pb, As, and MeHg) neurotoxicity: Deepening into the molecular mechanism of neurodegenerative diseases. J. Proteom. 2018, 187, 106–125. [Google Scholar] [CrossRef] [Green Version]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Jassam, Y.N.; Izzy, S.; Whalen, M.; McGavern, D.B.; El Khoury, J. Neuroimmunology of Traumatic Brain Injury: Time for a Paradigm Shift. Neuron 2017, 95, 1246–1265. [Google Scholar] [CrossRef] [Green Version]
- Petsko, G.A.; Small, S.A. Elucidating the causes of neurodegeneration. Science 2022, 377, 31–32. [Google Scholar] [CrossRef]
- Nimjee, S.M.; Sullenger, B.A. Therapeutic Aptamers: Evolving to Find their Clinical Niche. Curr. Med. Chem. 2020, 27, 4181–4193. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Aguzzi, A.; Barres, B.A.; Bennett, M.L. Microglia: Scapegoat, saboteur, or something else? Science 2013, 339, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Sabogal-Guáqueta, A.M.; Marmolejo-Garza, A.; de Pádua, V.P.; Eggen, B.; Boddeke, E.; Dolga, A.M. Microglia alterations in neurodegenerative diseases and their modeling with human induced pluripotent stem cell and other platforms. Prog. Neurobiol. 2020, 190, 101805. [Google Scholar] [CrossRef]
- Réu, P.; Khosravi, A.; Bernard, S.; Mold, J.E.; Salehpour, M.; Alkass, K.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; et al. The Lifespan and Turnover of Microglia in the Human Brain. Cell Rep. 2017, 20, 779–784. [Google Scholar] [CrossRef] [Green Version]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef] [Green Version]
- Askew, K.; Li, K.; Olmos-Alonso, A.; Garcia-Moreno, F.; Liang, Y.; Richardson, P.; Tipton, T.; Chapman, M.A.; Riecken, K.; Beccari, S.; et al. Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain. Cell Rep. 2017, 18, 391–405. [Google Scholar] [CrossRef] [Green Version]
- Priller, J.; Prinz, M. Targeting microglia in brain disorders. Science 2019, 365, 32–33. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Yan, W.; Gu, Z.; Li, Y.; Chen, L.; He, B. Anti-Neuroinflammatory Potential of Natural Products in the Treatment of Alzheimer’s Disease. Molecules 2023, 28, 1486. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, S.R.; Ayad, N.G.; Lee, J.K. Neuroinflammation Treatment via Targeted Delivery of Nanoparticles. Front. Cell. Neurosci. 2020, 14, 576037. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Chen, J.; Chu, F.; Zhu, J.; Jin, T. Inflammatory Role of TLR-MyD88 Signaling in Multiple Sclerosis. Front. Mol. Neurosci. 2019, 12, 314. [Google Scholar] [CrossRef]
- Vaure, C.; Liu, Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 2014, 5, 316. [Google Scholar] [CrossRef] [Green Version]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Kaur, J.; Singh, H.; Naqvi, S. Intracellular DAMPs in Neurodegeneration and Their Role in Clinical Therapeutics. Mol. Neurobiol. 2023, 60, 3600–3616. [Google Scholar] [CrossRef]
- Al-Ghraiybah, N.F.; Wang, J.; Alkhalifa, A.E.; Roberts, A.B.; Raj, R.; Yang, E.; Kaddoumi, A. Glial Cell-Mediated Neuroinflammation in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 10572. [Google Scholar] [CrossRef]
- Jung, E.S.; Suh, K.; Han, J.; Kim, H.; Kang, H.S.; Choi, W.S.; Mook-Jung, I. Amyloid-β activates NLRP3 inflammasomes by affecting microglial immunometabolism through the Syk-AMPK pathway. Aging Cell 2022, 21, e13623. [Google Scholar] [CrossRef]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation pathways: A general review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef]
- de Oliveira, J.; Kucharska, E.; Garcez, M.L.; Rodrigues, M.S.; Quevedo, J.; Moreno-Gonzalez, I.; Budni, J. Inflammatory Cascade in Alzheimer’s Disease Pathogenesis: A Review of Experimental Findings. Cells 2021, 10, 2581. [Google Scholar] [CrossRef]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Front. Pharmacol. 2019, 10, 1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco-Bocanegra, D.K.; Gourari, Y.; McAuley, C.; Chatelet, D.S.; Johnston, D.A.; Nicoll, J.A.R.; Boche, D. Microglial morphology in Alzheimer’s disease and after Aβ immunotherapy. Sci. Rep. 2021, 11, 15955. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Hussain, M.D.; Yan, L.J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 2014, 124, 307–321. [Google Scholar] [CrossRef]
- Masuda, T.; Sankowski, R.; Staszewski, O.; Böttcher, C.; Amann, L.; Sagar; Scheiwe, C.; Nessler, S.; Kunz, P.; van Loo, G.; et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 2019, 566, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.C.; Nakamura, M.C.; Hsieh, C.L. Brain trauma elicits non-canonical macrophage activation states. J. Neuroinflammation 2016, 13, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Dukay, B.; Csoboz, B.; Tóth, M.E. Heat-Shock Proteins in Neuroinflammation. Front. Pharmacol. 2019, 10, 920. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Zhou, T.; Sun, X.; Zheng, Y.; Cheng, B.; Li, M.; Liu, X.; He, C. Necroptosis in microglia contributes to neuroinflammation and retinal degeneration through TLR4 activation. Cell Death Differ. 2018, 25, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, A.F.; Davies, C.L.; Holloway, R.K.; Labrak, Y.; Ireland, G.; Carradori, D.; Dillenburg, A.; Borger, E.; Soong, D.; Richardson, J.C.; et al. Central nervous system regeneration is driven by microglia necroptosis and repopulation. Nat. Neurosci. 2019, 22, 1046–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [Green Version]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, M.F.; Gan, Z.Y.; Komander, D.; Dewson, G. Ubiquitin signalling in neurodegeneration: Mechanisms and therapeutic opportunities. Cell Death Differ. 2021, 28, 570–590. [Google Scholar] [CrossRef]
- Geloso, M.C.; D’Ambrosi, N. Microglial Pruning: Relevance for Synaptic Dysfunction in Multiple Sclerosis and Related Experimental Models. Cells 2021, 10, 686. [Google Scholar] [CrossRef]
- Voet, S.; Prinz, M.; van Loo, G. Microglia in Central Nervous System Inflammation and Multiple Sclerosis Pathology. Trends Mol. Med. 2019, 25, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ma, N.; Zhong, J.; Yu, B.; Wan, J.; Zhang, W. Age-associated changes in microglia and astrocytes ameliorate blood-brain barrier dysfunction. Mol. Ther. Nucleic Acids 2021, 26, 970–986. [Google Scholar] [CrossRef] [PubMed]
- Cooper-Knock, J.; Kirby, J.; Ferraiuolo, L.; Heath, P.R.; Rattray, M.; Shaw, P.J. Gene expression profiling in human neurodegenerative disease. Nat. Rev. Neurol. 2012, 8, 518–530. [Google Scholar] [CrossRef]
- McQuade, A.; Kang, Y.J.; Hasselmann, J.; Jairaman, A.; Sotelo, A.; Coburn, M.; Shabestari, S.K.; Chadarevian, J.P.; Fote, G.; Tu, C.H.; et al. Gene expression and functional deficits underlie TREM2-knockout microglia responses in human models of Alzheimer’s disease. Nat. Commun. 2020, 11, 5370. [Google Scholar] [CrossRef] [PubMed]
- Rangaraju, S.; Dammer, E.B.; Raza, S.A.; Rathakrishnan, P.; Xiao, H.; Gao, T.; Duong, D.M.; Pennington, M.W.; Lah, J.J.; Seyfried, N.T.; et al. Identification and therapeutic modulation of a pro-inflammatory subset of disease-associated-microglia in Alzheimer’s disease. Mol. Neurodegener. 2018, 13, 24. [Google Scholar] [CrossRef] [Green Version]
- van den Elsen, P.J.; van Eggermond, M.C.; Puentes, F.; van der Valk, P.; Baker, D.; Amor, S. The epigenetics of multiple sclerosis and other related disorders. Mult. Scler. Relat. Disord. 2014, 3, 163–175. [Google Scholar] [CrossRef]
- Ferreira, S.A.; Romero-Ramos, M. Microglia Response During Parkinson’s Disease: Alpha-Synuclein Intervention. Front. Cell. Neurosci. 2018, 12, 247. [Google Scholar] [CrossRef] [Green Version]
- Moore, A.H.; Bigbee, M.J.; Boynton, G.E.; Wakeham, C.M.; Rosenheim, H.M.; Staral, C.J.; Morrissey, J.L.; Hund, A.K. Non-Steroidal Anti-Inflammatory Drugs in Alzheimer’s Disease and Parkinson’s Disease: Reconsidering the Role of Neuroinflammation. Pharmaceuticals 2010, 3, 1812–1841. [Google Scholar] [CrossRef] [Green Version]
- Imbimbo, B.P. An update on the efficacy of non-steroidal anti-inflammatory drugs in Alzheimer’s disease. Expert Opin. Investig. Drugs 2009, 18, 1147–1168. [Google Scholar] [CrossRef]
- Ajmone-Cat, M.A.; Bernardo, A.; Greco, A.; Minghetti, L. Non-Steroidal Anti-Inflammatory Drugs and Brain Inflammation: Effects on Microglial Functions. Pharmaceuticals 2010, 3, 1949–1965. [Google Scholar] [CrossRef]
- Ali, M.M.; Ghouri, R.G.; Ans, A.H.; Akbar, A.; Toheed, A. Recommendations for Anti-inflammatory Treatments in Alzheimer’s Disease: A Comprehensive Review of the Literature. Cureus 2019, 11, e4620. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wang, Y.; Wang, D.; Zhang, J.; Zhang, F. NSAID Exposure and Risk of Alzheimer’s Disease: An Updated Meta-Analysis From Cohort Studies. Front. Aging Neurosci. 2018, 10, 83. [Google Scholar] [CrossRef]
- Rivers-Auty, J.; Mather, A.E.; Peters, R.; Lawrence, C.B.; Brough, D. Anti-inflammatories in Alzheimer’s disease—Potential therapy or spurious correlate? Brain Commun. 2020, 2, fcaa109. [Google Scholar] [CrossRef]
- Daniels, M.J.; Rivers-Auty, J.; Schilling, T.; Spencer, N.G.; Watremez, W.; Fasolino, V.; Booth, S.J.; White, C.S.; Baldwin, A.G.; Freeman, S.; et al. Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer’s disease in rodent models. Nat. Commun. 2016, 7, 12504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bascones-Martinez, A.; Mattila, R.; Gomez-Font, R.; Meurman, J.H. Immunomodulatory drugs: Oral and systemic adverse effects. Med. Oral. Patol. Oral. Cir. Bucal 2014, 19, e24–e31. [Google Scholar] [CrossRef] [PubMed]
- Sehr, T.; Proschmann, U.; Thomas, K.; Marggraf, M.; Straube, E.; Reichmann, H.; Chan, A.; Ziemssen, T. New insights into the pharmacokinetics and pharmacodynamics of natalizumab treatment for patients with multiple sclerosis, obtained from clinical and in vitro studies. J. Neuroinflammation 2016, 13, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clerico, M.; Artusi, C.A.; Liberto, A.D.; Rolla, S.; Bardina, V.; Barbero, P.; Mercanti, S.F.; Durelli, L. Natalizumab in Multiple Sclerosis: Long-Term Management. Int. J. Mol. Sci. 2017, 18, 940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, J.J.; Abu-Rub, M.; Miller, R.H. B Cells in Neuroinflammation: New Perspectives and Mechanistic Insights. Cells 2021, 10, 1605. [Google Scholar] [CrossRef]
- Gärtner, J.; Hauser, S.L.; Bar-Or, A.; Montalban, X.; Cohen, J.A.; Cross, A.H.; Deiva, K.; Ganjgahi, H.; Häring, D.A.; Li, B.; et al. Efficacy and safety of ofatumumab in recently diagnosed, treatment-naive patients with multiple sclerosis: Results from ASCLEPIOS I and II. Mult. Scler. J. 2022, 28, 1562–1575. [Google Scholar] [CrossRef] [PubMed]
- Tobinick, E. Perispinal etanercept for treatment of Alzheimers disease. Curr. Alzheimer Res. 2007, 4, 550–552. [Google Scholar] [CrossRef]
- Butchart, J.; Brook, L.; Hopkins, V.; Teeling, J.; Püntener, U.; Culliford, D.; Sharples, R.; Sharif, S.; McFarlane, B.; Raybould, R.; et al. Etanercept in Alzheimer disease: A randomized, placebo-controlled, double-blind, phase 2 trial. Neurology 2015, 84, 2161–2168. [Google Scholar] [CrossRef] [Green Version]
- Becher, B.; Spath, S.; Goverman, J. Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 2017, 17, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, S.S.; Suliman, R.S.; Aljammaz, N.A.; Kahtani, K.M.; Aljatli, D.A.; Albadrani, G.M. Natural Products as Novel Neuroprotective Agents; Computational Predictions of the Molecular Targets, ADME Properties, and Safety Profile. Plants 2022, 11, 549. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhou, Y.; Zhang, S.; Li, J.; Zheng, Y.; Fan, X. The natural (poly)phenols as modulators of microglia polarization via TLR4/NF-κB pathway exert anti-inflammatory activity in ischemic stroke. Eur. J. Pharmacol. 2022, 914, 174660. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.; Vengalasetti, Y.V.; Bredesen, D.E.; Rao, R.V. Neuroprotective Herbs for the Management of Alzheimer’s Disease. Biomolecules 2021, 11, 543. [Google Scholar] [CrossRef]
- Cheng, S.; Hou, J.; Zhang, C.; Xu, C.; Wang, L.; Zou, X.; Yu, H.; Shi, Y.; Yin, Z.; Chen, G. Minocycline reduces neuroinflammation but does not ameliorate neuron loss in a mouse model of neurodegeneration. Sci. Rep. 2015, 5, 10535. [Google Scholar] [CrossRef] [Green Version]
- Shal, B.; Ding, W.; Ali, H.; Kim, Y.S.; Khan, S. Anti-neuroinflammatory Potential of Natural Products in Attenuation of Alzheimer’s Disease. Front. Pharmacol. 2018, 9, 548. [Google Scholar] [CrossRef]
- Shin, J.A.; Lee, H.; Lim, Y.K.; Koh, Y.; Choi, J.H.; Park, E.M. Therapeutic effects of resveratrol during acute periods following experimental ischemic stroke. J. Neuroimmunol. 2010, 227, 93–100. [Google Scholar] [CrossRef]
- Yang, X.; Xu, S.; Qian, Y.; Xiao, Q. Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury. Brain Behav. Immun. 2017, 64, 162–172. [Google Scholar] [CrossRef]
- Gomes, B.A.Q.; Silva, J.P.B.; Romeiro, C.F.R.; Dos Santos, S.M.; Rodrigues, C.A.; Gonçalves, P.R.; Sakai, J.T.; Mendes, P.F.S.; Varela, E.L.P.; Monteiro, M.C. Neuroprotective Mechanisms of Resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxidative Med. Cell. Longev. 2018, 2018, 8152373. [Google Scholar] [CrossRef] [Green Version]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Kobayashi, K.; Imagama, S.; Ohgomori, T.; Hirano, K.; Uchimura, K.; Sakamoto, K.; Hirakawa, A.; Takeuchi, H.; Suzumura, A.; Ishiguro, N.; et al. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 2013, 4, e525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camargos, Q.M.; Silva, B.C.; Silva, D.G.; Toscano, E.C.B.; Oliveira, B.D.S.; Bellozi, P.M.Q.; Jardim, B.L.O.; Vieira, É.L.M.; de Oliveira, A.C.P.; Sousa, L.P.; et al. Minocycline treatment prevents depression and anxiety-like behaviors and promotes neuroprotection after experimental ischemic stroke. Brain Res. Bull. 2020, 155, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Derfuss, T.; Mehling, M.; Papadopoulou, A.; Bar-Or, A.; Cohen, J.A.; Kappos, L. Advances in oral immunomodulating therapies in relapsing multiple sclerosis. Lancet Neurol. 2020, 19, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhou, M.; Li, Y.; Li, Y.; Hua, Y.; Fan, Y. Minocycline promotes functional recovery in ischemic stroke by modulating microglia polarization through STAT1/STAT6 pathways. Biochem. Pharmacol. 2021, 186, 114464. [Google Scholar] [CrossRef]
- Wadhwa, M.; Prabhakar, A.; Ray, K.; Roy, K.; Kumari, P.; Jha, P.K.; Kishore, K.; Kumar, S.; Panjwani, U. Inhibiting the microglia activation improves the spatial memory and adult neurogenesis in rat hippocampus during 48 h of sleep deprivation. J. Neuroinflammation 2017, 14, 222. [Google Scholar] [CrossRef] [Green Version]
- Familian, A.; Boshuizen, R.S.; Eikelenboom, P.; Veerhuis, R. Inhibitory effect of minocycline on amyloid β fibril formation and human microglial activation. Glia 2006, 53, 233–240. [Google Scholar] [CrossRef]
- Tagliavini, F.; Forloni, G.; Colombo, L.; Rossi, G.; Girola, L.; Canciani, B.; Angeretti, N.; Giampaolo, L.; Peressini, E.; Awan, T.; et al. Tetracycline affects abnormal properties of synthetic PrP peptides and PrPSc in vitro. J. Mol. Biol. 2000, 300, 1309–1322. [Google Scholar] [CrossRef]
- Balducci, C.; Santamaria, G.; La Vitola, P.; Brandi, E.; Grandi, F.; Viscomi, A.R.; Beeg, M.; Gobbi, M.; Salmona, M.; Ottonello, S.; et al. Doxycycline counteracts neuroinflammation restoring memory in Alzheimer’s disease mouse models. Neurobiol. Aging 2018, 70, 128–139. [Google Scholar] [CrossRef]
- Sriram, K.; Miller, D.B.; O’Callaghan, J.P. Minocycline attenuates microglial activation but fails to mitigate striatal dopaminergic neurotoxicity: Role of tumor necrosis factor-alpha. J. Neurochem. 2006, 96, 706–718. [Google Scholar] [CrossRef]
- Kaur, N.; Chugh, H.; Sakharkar, M.K.; Dhawan, U.; Chidambaram, S.B.; Chandra, R. Neuroinflammation Mechanisms and Phytotherapeutic Intervention: A Systematic Review. ACS Chem. Neurosci. 2020, 11, 3707–3731. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zeng, Z.; Wan, Q.; Liu, X.; Qi, J.; Zu, Y. Targeted immunotherapy of triple-negative breast cancer by aptamer-engineered NK cells. Biomaterials 2022, 280, 121259. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Hernández, C.D.; Rodríguez-Martínez, G.; Cortés-Ramírez, S.A.; Morales-Pacheco, M.; Cruz-Burgos, M.; Losada-García, A.; Reyes-Grajeda, J.P.; González-Ramírez, I.; González-Covarrubias, V.; Camacho-Arroyo, I.; et al. Aptamers as Theragnostic Tools in Prostate Cancer. Biomolecules 2022, 12, 1056. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Nilsen-Hamilton, M.; Ilgu, M. Aptamer Applications in Neuroscience. Pharmaceuticals 2021, 14, 1260. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Zhuo, Z.; Pan, Y.; Yu, Y.; Li, F.; Liu, J.; Wang, L.; Wu, X.; Li, D.; Wan, Y.; et al. Recent Progress in Aptamer Discoveries and Modifications for Therapeutic Applications. ACS Appl. Mater. Interfaces 2021, 13, 9500–9519. [Google Scholar] [CrossRef]
- Sullivan, R.; Adams, M.C.; Naik, R.R.; Milam, V.T. Analyzing Secondary Structure Patterns in DNA Aptamers Identified via CompELS. Molecules 2019, 24, 1572. [Google Scholar] [CrossRef] [Green Version]
- Bernat, V.; Disney, M.D. RNA Structures as Mediators of Neurological Diseases and as Drug Targets. Neuron 2015, 87, 28–46. [Google Scholar] [CrossRef] [Green Version]
- Sefah, K.; Tang, Z.W.; Shangguan, D.H.; Chen, H.; Lopez-Colon, D.; Li, Y.; Parekh, P.; Martin, J.; Meng, L.; Phillips, J.A.; et al. Molecular recognition of acute myeloid leukemia using aptamers. Leukemia 2009, 23, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Duan, N.; Ding, X.; Wu, S.; Xia, Y.; Ma, X.; Wang, Z.; Chen, J. In vitro selection of a DNA aptamer targeted against Shigella dysenteriae. J. Microbiol. Methods 2013, 94, 170–174. [Google Scholar] [CrossRef]
- O’Donoghue, M.B.; Shi, X.; Fang, X.; Tan, W. Single-molecule atomic force microscopy on live cells compares aptamer and antibody rupture forces. Anal. Bioanal. Chem. 2012, 402, 3205–3209. [Google Scholar] [CrossRef]
- Fu, Z.; Xiang, J. Aptamers, the Nucleic Acid Antibodies, in Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 2793. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, T.; Oshima, H.; Mashima, T.; Nagata, T.; Katahira, M.; Kinoshita, M. Binding of an RNA aptamer and a partial peptide of a prion protein: Crucial importance of water entropy in molecular recognition. Nucleic Acids Res. 2014, 42, 6861–6875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, T.; Ennifar, E.; Nakamura, Y. Thermodynamic study of aptamers binding to their target proteins. Biochimie 2018, 145, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Lebars, I.; Legrand, P.; Aimé, A.; Pinaud, N.; Fribourg, S.; Di Primo, C. Exploring TAR–RNA aptamer loop–loop interaction by X-ray crystallography, UV spectroscopy and surface plasmon resonance. Nucleic Acids Res. 2008, 36, 7146–7156. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Wen, N.; Xiao, D.; Yan, J.; Xiong, H.; Cai, S.; Liu, Z.; Liu, Y. Aptamer-Based Targeted Drug Delivery Systems: Current Potential and Challenges. Curr. Med. Chem. 2020, 27, 2189–2219. [Google Scholar] [CrossRef]
- Chaturvedi, M.; Schilling, J.; Beautrait, A.; Bouvier, M.; Benovic, J.L.; Shukla, A.K. Emerging Paradigm of Intracellular Targeting of G Protein-Coupled Receptors. Trends Biochem. Sci. 2018, 43, 533–546. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, C.; Ma, T.; Liu, X.; Chen, Z.; Li, S.; Deng, Y. Advances in aptamer screening and aptasensors’ detection of heavy metal ions. J. Nanobiotechnology 2021, 19, 166. [Google Scholar] [CrossRef]
- Murakami, K.; Izuo, N.; Bitan, G. Aptamers targeting amyloidogenic proteins and their emerging role in neurodegenerative diseases. J. Biol. Chem. 2022, 298, 101478. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, S.; Wei, X.; Wan, S.; Huang, M.; Song, T.; Lu, Y.; Weng, X.; Lin, Z.; Chen, H.; et al. Aptamer Blocking Strategy Inhibits SARS-CoV-2 Virus Infection. Angew. Chem. Int. Ed. 2021, 60, 10266–10272. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, E.; Pan, Z.; Zhang, C.; Zhang, Y.; Liao, Q.; Wang, Y.; Sun, Y.; Ye, M.; Wu, W. Development of an Aptamer-Based Molecular Tool for Specifically Targeting Microglia via the CD64 Protein. Anal. Chem. 2023, 95, 3238–3246. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef]
- Gold, L.; Brown, D.; He, Y.; Shtatland, T.; Singer, B.S.; Wu, Y. From oligonucleotide shapes to genomic SELEX: Novel biological regulatory loops. Proc. Natl. Acad. Sci. USA 1997, 94, 59–64. [Google Scholar] [CrossRef]
- Ohuchi, S. Cell-SELEX Technology. Bioresearch Open Access 2012, 1, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Komarova, N.; Kuznetsov, A. Inside the Black Box: What Makes SELEX Better? Molecules 2019, 24, 3598. [Google Scholar] [CrossRef] [Green Version]
- Jayasena, S.D. Aptamers: An emerging class of molecules that rival antibodies in diagnostics. Clin. Chem. 1999, 45, 1628–1650. [Google Scholar] [CrossRef] [PubMed]
- Bayat, P.; Nosrati, R.; Alibolandi, M.; Rafatpanah, H.; Abnous, K.; Khedri, M.; Ramezani, M. SELEX methods on the road to protein targeting with nucleic acid aptamers. Biochimie 2018, 154, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yu, Y.; Jiang, F.; Zhou, J.; Li, Y.; Liang, C.; Dang, L.; Lu, A.; Zhang, G. Development of Cell-SELEX Technology and Its Application in Cancer Diagnosis and Therapy. Int. J. Mol. Sci. 2016, 17, 2079. [Google Scholar] [CrossRef] [Green Version]
- Lin, N.; Wu, L.; Xu, X.; Wu, Q.; Wang, Y.; Shen, H.; Song, Y.; Wang, H.; Zhu, Z.; Kang, D.; et al. Aptamer Generated by Cell-SELEX for Specific Targeting of Human Glioma Cells. ACS Appl. Mater. Interfaces 2021, 13, 9306–9315. [Google Scholar] [CrossRef]
- Song, Z.; Mao, J.; Barrero, R.A.; Wang, P.; Zhang, F.; Wang, T. Development of a CD63 Aptamer for Efficient Cancer Immunochemistry and Immunoaffinity-Based Exosome Isolation. Molecules 2020, 25, 5585. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, C.; Wang, Y.; Chen, G. Research progress of whole-cell-SELEX selection and the application of cell-targeting aptamer. Mol. Biol. Rep. 2022, 49, 7979–7993. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-Y.; Choi, J.-H.; Lim, J.; Lee, S.-N.; Choi, J.-W. Microfluidic Chip-Based Cancer Diagnosis and Prediction of Relapse by Detecting Circulating Tumor Cells and Circulating Cancer Stem Cells. Cancers 2021, 13, 1385. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Hemmat, M.A.; Shubham, S.; Gosai, A.; Devarakonda, S.; Jiang, N.; Geekiyanage, C.; Dillard, J.A.; Maury, W.; Shrotriya, P.; et al. Structurally Different Yet Functionally Similar: Aptamers Specific for the Ebola Virus Soluble Glycoprotein and GP1,2 and Their Application in Electrochemical Sensing. Int. J. Mol. Sci. 2023, 24, 4627. [Google Scholar] [CrossRef] [PubMed]
- Guo, P. The emerging field of RNA nanotechnology. Nat. Nanotechnol. 2010, 5, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, S.; Yao, H.; Wang, L.; Lu, J.; Jiang, F.; Lu, A.; Zhang, G. Chemical Modifications of Nucleic Acid Aptamers for Therapeutic Purposes. Int. J. Mol. Sci. 2017, 18, 1683. [Google Scholar] [CrossRef]
- Odeh, F.; Nsairat, H.; Alshaer, W.; Ismail, M.A.; Esawi, E.; Qaqish, B.; Bawab, A.A.; Ismail, S.I. Aptamers Chemistry: Chemical Modifications and Conjugation Strategies. Molecules 2019, 25, 3. [Google Scholar] [CrossRef] [Green Version]
- Ji, D.; Lyu, K.; Zhao, H.; Kwok, C.K. Circular L-RNA aptamer promotes target recognition and controls gene activity. Nucleic Acids Res. 2021, 49, 7280–7291. [Google Scholar] [CrossRef]
- Ma, P.; Ye, H.; Guo, H.; Ma, X.; Yue, L.; Wang, Z. Aptamer truncation strategy assisted by molecular docking and sensitive detection of T-2 toxin using SYBR Green I as a signal amplifier. Food Chem. 2022, 381, 132171. [Google Scholar] [CrossRef]
- Aljohani, M.M.; Cialla-May, D.; Popp, J.; Chinnappan, R.; Al-Kattan, K.; Zourob, M. Aptamers: Potential Diagnostic and Therapeutic Agents for Blood Diseases. Molecules 2022, 27, 383. [Google Scholar] [CrossRef]
- Zhu, G.; Niu, G.; Chen, X. Aptamer-Drug Conjugates. Bioconjugate Chem. 2015, 26, 2186–2197. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.; Chen, X. Aptamer-based targeted therapy. Adv. Drug Deliv. Rev. 2018, 134, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Doherty, G.J.; McMahon, H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMahon, H.T.; Boucrot, E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2011, 12, 517–533. [Google Scholar] [CrossRef]
- Alamudi, S.H.; Kimoto, M.; Hirao, I. Uptake mechanisms of cell-internalizing nucleic acid aptamers for applications as pharmacological agents. RSC Med. Chem. 2021, 12, 1640–1649. [Google Scholar] [CrossRef]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef]
- Wan, L.-Y.; Yuan, W.-F.; Ai, W.-B.; Ai, Y.-W.; Wang, J.-J.; Chu, L.-Y.; Zhang, Y.-Q.; Wu, J.-F. An exploration of aptamer internalization mechanisms and their applications in drug delivery. Expert Opin. Drug Deliv. 2019, 16, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, Q.; Zhang, H.; Deng, T.; Feng, P.; Hu, B.; Jiang, Y.; Cao, L. Characterization of a DNA Aptamer for Ovarian Cancer Clinical Tissue Recognition and in Vivo Imaging. Cell. Physiol. Biochem. 2018, 51, 2564–2574. [Google Scholar] [CrossRef]
- Bukari, B.; Samarasinghe, R.M.; Noibanchong, J.; Shigdar, S.L. Non-Invasive Delivery of Therapeutics into the Brain: The Potential of Aptamers for Targeted Delivery. Biomedicines 2020, 8, 120. [Google Scholar] [CrossRef]
- Ilgu, M.; Nilsen-Hamilton, M. Aptamers in analytics. Analyst 2016, 141, 1551–1568. [Google Scholar] [CrossRef] [Green Version]
- Lincoff, A.M.; Mehran, R.; Povsic, T.J.; Zelenkofske, S.L.; Huang, Z.; Armstrong, P.W.; Steg, P.G.; Bode, C.; Cohen, M.G.; Buller, C.; et al. Effect of the REG1 anticoagulation system versus bivalirudin on outcomes after percutaneous coronary intervention (REGULATE-PCI): A randomised clinical trial. Lancet 2016, 387, 349–356. [Google Scholar] [CrossRef]
- Andersson, P.; den Besten, C. CHAPTER 20 Preclinical and Clinical Drug-metabolism, Pharmacokinetics and Safety of Therapeutic Oligonucleotides. In Advances in Nucleic Acid Therapeutics; The Royal Society of Chemistry: London, UK, 2019; pp. 474–531. [Google Scholar] [CrossRef]
- Hammond, S.M.; Aartsma-Rus, A.; Alves, S.; Borgos, S.E.; Buijsen, R.A.M.; Collin, R.W.J.; Covello, G.; Denti, M.A.; Desviat, L.R.; Echevarría, L.; et al. Delivery of oligonucleotide-based therapeutics: Challenges and opportunities. EMBO Mol. Med. 2021, 13, e13243. [Google Scholar] [CrossRef] [PubMed]
- Healy, J.M.; Lewis, S.D.; Kurz, M.; Boomer, R.M.; Thompson, K.M.; Wilson, C.; McCauley, T.G. Pharmacokinetics and biodistribution of novel aptamer compositions. Pharm. Res. 2004, 21, 2234–2246. [Google Scholar] [CrossRef]
- Godfrey, C.; Desviat, L.R.; Smedsrød, B.; Piétri-Rouxel, F.; Denti, M.A.; Disterer, P.; Lorain, S.; Nogales-Gadea, G.; Sardone, V.; Anwar, R.; et al. Delivery is key: Lessons learnt from developing splice-switching antisense therapies. EMBO Mol. Med. 2017, 9, 545–557. [Google Scholar] [CrossRef]
- Wang, H.; Su, Y.; Chen, D.; Li, Q.; Shi, S.; Huang, X.; Fang, M.; Yang, M. Advances in the mechanisms and applications of inhibitory oligodeoxynucleotides against immune-mediated inflammatory diseases. Front. Pharmacol. 2023, 14, 1119431. [Google Scholar] [CrossRef] [PubMed]
- Hoellenriegel, J.; Zboralski, D.; Maasch, C.; Rosin, N.Y.; Wierda, W.G.; Keating, M.J.; Kruschinski, A.; Burger, J.A. The Spiegelmer NOX-A12, a novel CXCL12 inhibitor, interferes with chronic lymphocytic leukemia cell motility and causes chemosensitization. Blood 2014, 123, 1032–1039. [Google Scholar] [CrossRef]
- Kreuter, J. Influence of the surface properties on nanoparticle-mediated transport of drugs to the brain. J. Nanosci. Nanotechnol. 2004, 4, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Tischner, D.; Reichardt, H.M. Glucocorticoids in the control of neuroinflammation. Mol. Cell. Endocrinol. 2007, 275, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef]
- Oray, M.; Abu Samra, K.; Ebrahimiadib, N.; Meese, H.; Foster, C.S. Long-term side effects of glucocorticoids. Expert Opin. Drug Saf. 2016, 15, 457–465. [Google Scholar] [CrossRef]
- Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Front. Immunol. 2019, 10, 1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migliorati, J.M.; Liu, S.; Liu, A.; Gogate, A.; Nair, S.; Bahal, R.; Rasmussen, T.P.; Manautou, J.E.; Zhong, X.-B. Absorption, Distribution, Metabolism, and Excretion of US Food and Drug Administration–Approved Antisense Oligonucleotide Drugs. Drug Metab. Dispos. 2022, 50, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Sun, W.; Fu, T.; Liu, X.; Chen, P.; Qiu, L.; Qu, F.; Tan, W. Aptamer-Based Targeted Delivery of Functional Nucleic Acids. J. Am. Chem. Soc. 2023, 145, 7677–7691. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Preda, M.D.; Niculescu, A.G.; Vladâcenco, O.; Radu, C.I.; Grumezescu, A.M.; Teleanu, D.M. Current Strategies to Enhance Delivery of Drugs across the Blood-Brain Barrier. Pharmaceutics 2022, 14, 987. [Google Scholar] [CrossRef] [PubMed]
- Bregy, A.; Shah, A.H.; Diaz, M.V.; Pierce, H.E.; Ames, P.L.; Diaz, D.; Komotar, R.J. The role of Gliadel wafers in the treatment of high-grade gliomas. Expert Rev. Anticancer. Ther. 2013, 13, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-T.; Zhao, Y.-Z.; Wong, H.L.; Cai, J.; Peng, L.; Tian, X.-Q. Current approaches to enhance CNS delivery of drugs across the brain barriers. Int. J. Nanomedicine 2014, 9, 2241–2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardridge, W.M. Drug transport in brain via the cerebrospinal fluid. Fluids Barriers CNS 2011, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, S.; Luo, Y.; Han, Z. Crosstalk between Inflammation and the BBB in Stroke. Curr. Neuropharmacol. 2020, 18, 1227–1236. [Google Scholar] [CrossRef]
- Fu, B.M.; Zhao, Z.; Zhu, D. Blood-Brain Barrier (BBB) Permeability and Transport Measurement In Vitro and In Vivo. Methods Mol. Biol. 2021, 2367, 105–122. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Furtado, D.; Björnmalm, M.; Ayton, S.; Bush, A.I.; Kempe, K.; Caruso, F. Overcoming the Blood–Brain Barrier: The Role of Nanomaterials in Treating Neurological Diseases. Adv. Mater. 2018, 30, e1801362. [Google Scholar] [CrossRef] [Green Version]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Bellettato, C.M.; Scarpa, M. Possible strategies to cross the blood–brain barrier. Ital. J. Pediatr. 2018, 44 (Suppl. S2), 131. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Choi, K.; Kim, D.-H.; Oh, B.-K.; Yim, H.; Jo, S.; Choi, C. Strategies for Targeted Delivery of Exosomes to the Brain: Advantages and Challenges. Pharmaceutics 2022, 14, 672. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Chen, H.; Wang, L.; Zeng, X.; Schwarz, H.; Nanda, H.S.; Peng, X.; Zhou, Y. Exosomes, a New Star for Targeted Delivery. Front. Cell Dev. Biol. 2021, 9, 751079. [Google Scholar] [CrossRef]
- Zheng, Y.; Qu, J.; Xue, F.; Zheng, Y.; Yang, B.; Chang, Y.; Yang, H.; Zhang, J. Novel DNA Aptamers for Parkinson’s Disease Treatment Inhibit α-Synuclein Aggregation and Facilitate its Degradation. Mol. Ther. Nucleic Acids 2018, 11, 228–242. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Zhao, Y.; Xue, F.; Zheng, Y.; Huang, H.; Wang, W.; Chang, Y.; Yang, H.; Zhang, J. Exosomal DNA Aptamer Targeting α-Synuclein Aggregates Reduced Neuropathological Deficits in a Mouse Parkinson’s Disease Model. Mol. Ther. Nucleic Acids 2019, 17, 726–740. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Chen, Y.H.; Lennox, K.A.; Behlke, M.A.; Davidson, B.L. In vivo SELEX for Identification of Brain-penetrating Aptamers. Mol. Ther. Nucleic Acids 2013, 2, e67. [Google Scholar] [CrossRef] [PubMed]
- Wilner, S.E.; Wengerter, B.; Maier, K.; de Lourdes Borba Magalhães, M.; Del Amo, D.S.; Pai, S.; Opazo, F.; Rizzoli, S.O.; Yan, A.; Levy, M. An RNA alternative to human transferrin: A new tool for targeting human cells. Mol. Ther. Nucleic Acids 2012, 1, e21. [Google Scholar] [CrossRef]
- Chen, C.H.; Dellamaggiore, K.R.; Ouellette, C.P.; Sedano, C.D.; Lizadjohry, M.; Chernis, G.A.; Gonzales, M.; Baltasar, F.E.; Fan, A.L.; Myerowitz, R.; et al. Aptamer-based endocytosis of a lysosomal enzyme. Proc. Natl. Acad. Sci. USA 2008, 105, 15908–15913. [Google Scholar] [CrossRef] [PubMed]
- Kusmierz, C.D.; Callmann, C.E.; Kudruk, S.; Distler, M.E.; Mirkin, C.A. Transferrin Aptamers Increase the In Vivo Blood–Brain Barrier Targeting of Protein Spherical Nucleic Acids. Bioconjug Chem. 2022, 33, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, J.; Shen, L.; Wang, T.; Yang, J.; Li, Y.; Wang, Y.; Quan, D. Brain-targeted delivery of obidoxime, using aptamer-modified liposomes, for detoxification of organophosphorus compounds. J. Control. Release 2021, 329, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.; Zhao, H.; Zhu, T.; Yang, Z.; Xu, H.; Fu, Y.; Lin, F.; Pan, X.; Li, L.; et al. Enhanced in Vivo Blood–Brain Barrier Penetration by Circular Tau–Transferrin Receptor Bifunctional Aptamer for Tauopathy Therapy. J. Am. Chem. Soc. 2020, 142, 3862–3872. [Google Scholar] [CrossRef]
- Macdonald, J.; Henri, J.; Goodman, L.; Xiang, D.; Duan, W.; Shigdar, S. Development of a Bifunctional Aptamer Targeting the Transferrin Receptor and Epithelial Cell Adhesion Molecule (EpCAM) for the Treatment of Brain Cancer Metastases. ACS Chem. Neurosci. 2017, 8, 777–784. [Google Scholar] [CrossRef]
- Marsh, S.E.; Blurton-Jones, M. Examining the mechanisms that link β-amyloid and α-synuclein pathologies. Alzheimer’s Res. Ther. 2012, 4, 11. [Google Scholar] [CrossRef]
- Si, Z.-Z.; Zou, C.-J.; Mei, X.; Li, X.-F.; Luo, H.; Shen, Y.; Hu, J.; Li, X.-X.; Wu, L.; Liu, Y. Targeting neuroinflammation in Alzheimer’s disease: From mechanisms to clinical applications. Neural Regen. Res. 2023, 18, 708–715. [Google Scholar] [CrossRef]
- Song, M.K.; Lee, J.H.; Kim, J.; Kim, J.H.; Hwang, S.; Kim, Y.-S.; Kim, Y.-J. Neuroprotective effect of NXP031 in the MPTP-induced Parkinson’s disease model. Neurosci. Lett. 2021, 740, 135425. [Google Scholar] [CrossRef]
- Candia, J.; Cheung, F.; Kotliarov, Y.; Fantoni, G.; Sellers, B.; Griesman, T.; Huang, J.; Stuccio, S.; Zingone, A.; Ryan, B.M.; et al. Assessment of Variability in the SOMAscan Assay. Sci. Rep. 2017, 7, 14248. [Google Scholar] [CrossRef] [Green Version]
- Barbour, C.; Kosa, P.; Komori, M.; Tanigawa, M.; Masvekar, R.; Wu, T.; Johnson, K.; Douvaras, P.; Fossati, V.; Herbst, R.; et al. Molecular-based diagnosis of multiple sclerosis and its progressive stage. Ann. Neurol. 2017, 82, 795–812. [Google Scholar] [CrossRef] [PubMed]
- Timsina, J.; Gomez-Fonseca, D.; Wang, L.; Do, A.; Western, D.; Alvarez, I.; Aguilar, M.; Pastor, P.; Henson, R.L.; Herries, E.; et al. Comparative Analysis of Alzheimer’s Disease Cerebrospinal Fluid Biomarkers Measurement by Multiplex SOMAscan Platform and Immunoassay-Based Approach. J. Alzheimer’s Dis. 2022, 89, 193–207. [Google Scholar] [CrossRef]
- Kim, J.; Noh, S.; Park, J.A.; Park, S.-C.; Park, S.J.; Lee, J.-H.; Ahn, J.-H.; Lee, T. Recent Advances in Aptasensor for Cytokine Detection: A Review. Sensors 2021, 21, 8491. [Google Scholar] [CrossRef] [PubMed]
- Hun, X.; Kong, X. An enzyme linked aptamer photoelectrochemical biosensor for Tau-381 protein using AuNPs/MoSe2 as sensing material. J. Pharm. Biomed. Anal. 2021, 192, 113666. [Google Scholar] [CrossRef] [PubMed]
- Giorgi-Coll, S.; Marín, M.J.; Sule, O.; Hutchinson, P.J.; Carpenter, K.L.H. Aptamer-modified gold nanoparticles for rapid aggregation-based detection of inflammation: An optical assay for interleukin-6. Microchim. Acta 2019, 187, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, P.S.; Vaughan, E.; Islam, J.; Burke, N.; Iacopino, D.; Tierney, J.B. Laser Scribing Fabrication of Graphitic Carbon Biosensors for Label-Free Detection of Interleukin-6. Nanomaterials 2021, 11, 2110. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, M.; Zhang, D.; Li, P.; Wang, D.; Sun, S.; Wei, W. Detection of β-amyloid peptide aggregates by quartz crystal microbalance based on dual-aptamer assisted signal amplification. Anal. Chim. Acta 2023, 1244, 340857. [Google Scholar] [CrossRef]
- Mollasalehi, N.; Francois-Moutal, L.; Porciani, D.; Burke, D.H.; Khanna, M. Aptamers Targeting Hallmark Proteins of Neurodegeneration. Nucleic Acid Ther. 2022, 32, 235–250. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [Green Version]
- Obata, Y.; Murakami, K.; Kawase, T.; Hirose, K.; Izuo, N.; Shimizu, T.; Irie, K. Detection of Amyloid β Oligomers with RNA Aptamers in AppNL-G-F/NL-G-F Mice: A Model of Arctic Alzheimer’s Disease. ACS Omega 2020, 5, 21531–21537. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Obata, Y.; Sekikawa, A.; Ueda, H.; Izuo, N.; Awano, T.; Takabe, K.; Shimizu, T.; Irie, K. An RNA aptamer with potent affinity for a toxic dimer of amyloid β42 has potential utility for histochemical studies of Alzheimer’s disease. J. Biol. Chem. 2020, 295, 4870–4880. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Shi, Y.; Kou, Z.; Peng, Y.; Chen, W.; Li, X.; Li, S.; Wang, Y.; Wang, F.; Zhang, X. Inhibition of BACE1 Activity by a DNA Aptamer in an Alzheimer’s Disease Cell Model. PLoS ONE 2015, 10, e0140733. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.-M.; Peng, Y.-H.; Chen, Y.; Long, L.-L.; Luo, H.-J.; Chen, Y.-J.; Liang, Y.-L.; Tian, Y.-H.; Li, S.-J.; Shi, Y.-S.; et al. The BACE1-Specific DNA Aptamer A1 Rescues Amyloid-β Pathology and Behavioral Deficits in a Mouse Model of Alzheimer’s Disease. Nucleic Acid Ther. 2019, 29, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Tsukakoshi, K.; Abe, K.; Sode, K.; Ikebukuro, K. Selection of DNA aptamers that recognize α-synuclein oligomers using a competitive screening method. Anal. Chem. 2012, 84, 5542–5547. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, F.; Seo, H.; Javaid, N.; Kim, M.S.; Choi, S. Therapeutic Interventions into Innate Immune Diseases by Means of Aptamers. Pharmaceutics 2020, 12, 955. [Google Scholar] [CrossRef] [PubMed]
- García Melián, M.F.; Moreno, M.; Cerecetto, H.; Calzada, V. Aptamer-Based Immunotheranostic Strategies. Cancer Biother. Radiopharm. 2023, 38, 246–255. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, S.; Wang, Y.; Li, L.; Li, Y.; Yuan, D.; Luo, F.; Zhao, J.; Song, X.; Zhao, Y. Aptamer blocking S-TLR4 interaction selectively inhibits SARS-CoV-2 induced inflammation. Signal Transduct. Target. Ther. 2022, 7, 120. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, S.; Zhou, J.; Wang, C.; Li, K.; Liu, J.; Tang, Y.; Wang, L. Application of aptamers in regenerative medicine. Front. Bioeng. Biotechnol. 2022, 10, 976960. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, Z.; Jiang, J.; Piao, Y.; Li, L.; Xu, C.; Piao, H.; Li, L.; Yan, G. DEK-targeting aptamer DTA-64 attenuates bronchial EMT-mediated airway remodelling by suppressing TGF-β1/Smad, MAPK and PI3K signalling pathway in asthma. J. Cell. Mol. Med. 2020, 24, 13739–13750. [Google Scholar] [CrossRef] [PubMed]
- Orava, E.W.; Jarvik, N.; Shek, Y.L.; Sidhu, S.S.; Gariépy, J. A short DNA aptamer that recognizes TNFα and blocks its activity in vitro. ACS Chem. Biol. 2013, 8, 170–178. [Google Scholar] [CrossRef]
- Boshtam, M.; Asgary, S.; Kouhpayeh, S.; Shariati, L.; Khanahmad, H. Aptamers against Pro- and Anti-Inflammatory Cytokines: A Review. Inflammation 2017, 40, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Mor-Vaknin, N.; Saha, A.; Legendre, M.; Carmona-Rivera, C.; Amin, M.A.; Rabquer, B.J.; Gonzales-Hernandez, M.J.; Jorns, J.; Mohan, S.; Yalavarthi, S.; et al. DEK-targeting DNA aptamers as therapeutics for inflammatory arthritis. Nat. Commun. 2017, 8, 14252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Su, J.; An, M.; Yang, Y.; Zhang, Y.; Zuo, J.; Zhang, N.; Zhao, Y. Novel DEK-Targeting Aptamer Delivered by a Hydrogel Microneedle Attenuates Collagen-Induced Arthritis. Mol. Pharm. 2021, 18, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Shahdadi Sardou, H.; Jebali, A.; Iman, M. Dual function of interleukin-23 Aptamer to suppress brain inflammation via attachment to macrophage stimulating 1 kinase and interleukin-23. Colloids Surf. B Biointerfaces 2020, 185, 110619. [Google Scholar] [CrossRef]
- Simon, E.; Obst, J.; Gomez-Nicola, D. The Evolving Dialogue of Microglia and Neurons in Alzheimer’s Disease: Microglia as Necessary Transducers of Pathology. Neuroscience 2019, 405, 24–34. [Google Scholar] [CrossRef]
- Prodeus, A.; Cydzik, M.; Abdul-Wahid, A.; Huang, E.; Khatri, I.; Gorczynski, R.; Gariépy, J. Agonistic CD200R1 DNA Aptamers Are Potent Immunosuppressants That Prolong Allogeneic Skin Graft Survival. Mol. Ther. Nucleic Acids 2014, 3, e190. [Google Scholar] [CrossRef]
- Prodeus, A.; Sparkes, A.; Fischer, N.W.; Cydzik, M.; Huang, E.; Khatri, I.; Young, A.; Woo, L.; Chow, C.W.; Gorczynski, R.; et al. A Synthetic Cross-Species CD200R1 Agonist Suppresses Inflammatory Immune Responses In Vivo. Mol. Ther. Nucleic Acids 2018, 12, 350–358. [Google Scholar] [CrossRef] [Green Version]
- McNamara, J.O.; Kolonias, D.; Pastor, F.; Mittler, R.S.; Chen, L.; Giangrande, P.H.; Sullenger, B.; Gilboa, E. Multivalent 4-1BB binding aptamers costimulate CD8+ T cells and inhibit tumor growth in mice. J. Clin. Investig. 2008, 118, 376–386. [Google Scholar] [CrossRef] [Green Version]
- Reali, C.; Curto, M.; Sogos, V.; Scintu, F.; Pauly, S.; Schwarz, H.; Gremo, F. Expression of CD137 and its ligand in human neurons, astrocytes, and microglia: Modulation by FGF-2. J. Neurosci. Res. 2003, 74, 67–73. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [Green Version]
- Longbrake, E.E.; Racke, M.K. Why did IL-12/IL-23 antibody therapy fail in multiple sclerosis? Expert Rev. Neurother. 2009, 9, 319–321. [Google Scholar] [CrossRef]
- Nitsch, L.; Schneider, L.; Zimmermann, J.; Müller, M. Microglia-Derived Interleukin 23: A Crucial Cytokine in Alzheimer’s Disease? Front. Neurol. 2021, 12, 639353. [Google Scholar] [CrossRef]
- Eede, P.; Obst, J.; Benke, E.; Yvon-Durocher, G.; Richard, B.C.; Gimber, N.; Schmoranzer, J.; Böddrich, A.; Wanker, E.E.; Prokop, S.; et al. Interleukin-12/23 deficiency differentially affects pathology in male and female Alzheimer’s disease-like mice. EMBO Rep. 2020, 21, e48530. [Google Scholar] [CrossRef]
- Zhao, S.; Yin, J.; Zhou, L.; Yan, F.; He, Q.; Huang, L.; Peng, S.; Jia, J.; Cheng, J.; Chen, H.; et al. Hippo/MST1 signaling mediates microglial activation following acute cerebral ischemia–reperfusion injury. Brain Behav. Immun. 2016, 55, 236–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, S.; Doering, A.; Letiembre, M.; Liu, Y.; Hao, W.; Diem, R.; Bernreuther, C.; Glatzel, M.; Engelhardt, B.; Fassbender, K. The LPS receptor, CD14 in experimental autoimmune encephalomyelitis and multiple sclerosis. Cell. Physiol. Biochem. 2006, 17, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Heidari, A.; Yazdanpanah, N.; Rezaei, N. The role of Toll-like receptors and neuroinflammation in Parkinson’s disease. J. Neuroinflammation 2022, 19, 135. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Batista, C.R.A.; Saliba, S.W.; Yousif, N.M.; de Oliveira, A.C.P. Role of Microglia TLRs in Neurodegeneration. Front. Cell. Neurosci. 2018, 12, 329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blank, M.; Enzlein, T.; Hopf, C. LPS-induced lipid alterations in microglia revealed by MALDI mass spectrometry-based cell fingerprinting in neuroinflammation studies. Sci. Rep. 2022, 12, 2908. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Kao, W.-C.; Wang, W.-Y.; Wang, W.-Y.; Yang, R.-B.; Peck, K. Identification and characterization of oligonucleotides that inhibit Toll-like receptor 2-associated immune responses. Faseb J. 2009, 23, 3078–3088. [Google Scholar] [CrossRef] [PubMed]
- Stoll, H.; Steinle, H.; Wilhelm, N.; Hann, L.; Kunnakattu, S.-J.; Narita, M.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Rapid Complexation of Aptamers by Their Specific Antidotes. Molecules 2017, 22, 954. [Google Scholar] [CrossRef] [Green Version]
- Calvo-Rodriguez, M.; García-Rodríguez, C.; Villalobos, C.; Núñez, L. Role of Toll Like Receptor 4 in Alzheimer’s Disease. Front. Immunol. 2020, 11, 1588. [Google Scholar] [CrossRef] [PubMed]
- Bsibsi, M.; Ravid, R.; Gveric, D.; van Noort, J.M. Broad expression of Toll-like receptors in the human central nervous system. J. Neuropathol. Exp. Neurol. 2002, 61, 1013–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V. Toll-like receptors in the pathogenesis of neuroinflammation. J. Neuroimmunol. 2019, 332, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Mowry, F.E.; Peaden, S.C.; Stern, J.E.; Biancardi, V.C. TLR4 and AT1R mediate blood-brain barrier disruption, neuroinflammation, and autonomic dysfunction in spontaneously hypertensive rats. Pharmacol. Res. 2021, 174, 105877. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Jiménez, M.; Martín-Vílchez, S.; Ochoa, D.; Mejía-Abril, G.; Román, M.; Camargo-Mamani, P.; Luquero-Bueno, S.; Jilma, B.; Moro, M.A.; Fernández, G.; et al. First-in-human phase I clinical trial of a TLR4-binding DNA aptamer, ApTOLL: Safety and pharmacokinetics in healthy volunteers. Mol. Ther. Nucleic Acids 2022, 28, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Jiménez, M.; Abad-Santos, F.; Cotgreave, I.; Gallego, J.; Jilma, B.; Flores, A.; Jovin, T.G.; Vivancos, J.; Molina, C.A.; Montaner, J.; et al. APRIL: A double-blind, placebo-controlled, randomized, Phase Ib/IIa clinical study of ApTOLL for the treatment of acute ischemic stroke. Front. Neurol. 2023, 14, 1127585. [Google Scholar] [CrossRef]
- Zelek, W.M.; Morgan, B.P. Targeting complement in neurodegeneration: Challenges, risks, and strategies. Trends Pharmacol. Sci. 2022, 43, 615–628. [Google Scholar] [CrossRef]
- Li, S.; Jiang, D.; Rosenkrans, Z.T.; Barnhart, T.E.; Ehlerding, E.B.; Ni, D.; Engle, J.W.; Cai, W. Aptamer-Conjugated Framework Nucleic Acids for the Repair of Cerebral Ischemia-Reperfusion Injury. Nano Lett. 2019, 19, 7334–7341. [Google Scholar] [CrossRef]
- Zanotta, D.; Puricelli, S.; Bonoldi, G. Cognitive effects of a dietary supplement made from extract of Bacopa monnieri, astaxanthin, phosphatidylserine, and vitamin E in subjects with mild cognitive impairment: A noncomparative, exploratory clinical study. Neuropsychiatr. Dis. Treat. 2014, 10, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Gao, Q.; Deng, R.; Zeng, L.; Guo, J.; Ye, B.; Yu, J.; Guo, X. Aptamer engineering exosomes loaded on biomimetic periosteum to promote angiogenesis and bone regeneration by targeting injured nerves via JNK3 MAPK pathway. Mater. Today Bio 2022, 16, 100434. [Google Scholar] [CrossRef]
- Vavvas, D.; D’Amico, D.J. Pegaptanib (Macugen): Treating neovascular age-related macular degeneration and current role in clinical practice. Ophthalmol. Clin. N. Am. 2006, 19, 353–360. [Google Scholar] [CrossRef]
- Yazdian-Robati, R.; Bayat, P.; Oroojalian, F.; Zargari, M.; Ramezani, M.; Taghdisi, S.M.; Abnous, K. Therapeutic applications of AS1411 aptamer, an update review. Int. J. Biol. Macromol. 2020, 155, 1420–1431. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Bambury, R.M.; Van Allen, E.M.; Drabkin, H.A.; Lara, P.N., Jr.; Harzstark, A.L.; Wagle, N.; Figlin, R.A.; Smith, G.W.; Garraway, L.A.; et al. A phase II trial of AS1411 (a novel nucleolin-targeted DNA aptamer) in metastatic renal cell carcinoma. Investig. New Drugs 2014, 32, 178–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Sun, L.; Wu, Y.; Zhang, L.; Yang, Z. Bioactivity of 2’-deoxyinosine-incorporated aptamer AS1411. Sci. Rep. 2016, 6, 25799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Chen, X.; Yan, R.; Jiang, Z.; Zhou, B.; Lv, B. G-quadruplex-containing oligodeoxynucleotides as DNA topoisomerase I inhibitors. Int. J. Biol. Macromol. 2022, 223 Pt A, 281–289. [Google Scholar] [CrossRef]
- Mortada, I.; Farah, R.; Nabha, S.; Ojcius, D.M.; Fares, Y.; Almawi, W.Y.; Sadier, N.S. Immunotherapies for Neurodegenerative Diseases. Front. Neurol. 2021, 12, 654739. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Schafer, D.; Vincent, A.; Blachère, N.E.; Bar-Or, A. Neuroinflammation: Ways in Which the Immune System Affects the Brain. Neurotherapeutics 2015, 12, 896–909. [Google Scholar] [CrossRef] [Green Version]
- Lai, W.-Y.; Huang, B.-T.; Wang, J.-W.; Lin, P.-Y.; Yang, P.-C. A Novel PD-L1-targeting Antagonistic DNA Aptamer With Antitumor Effects. Mol. Ther. Nucleic Acids 2016, 5, e397. [Google Scholar] [CrossRef] [Green Version]
- Baeck, C.; Wehr, A.; Karlmark, K.R.; Heymann, F.; Vucur, M.; Gassler, N.; Huss, S.; Klussmann, S.; Eulberg, D.; Luedde, T.; et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut 2012, 61, 416–426. [Google Scholar] [CrossRef]
- Choi, S.; Han, J.; Kim, J.H.; Kim, A.R.; Kim, S.H.; Lee, W.; Yoon, M.Y.; Kim, G.; Kim, Y.S. Advances in dermatology using DNA aptamer “Aptamin C” innovation: Oxidative stress prevention and effect maximization of vitamin C through antioxidation. J. Cosmet. Dermatol. 2020, 19, 970–976. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-M.; Lee, J.H.; Song, M.K.; Kim, Y.-J. NXP032 Ameliorates Aging-Induced Oxidative Stress and Cognitive Impairment in Mice through Activation of Nrf2 Signaling. Antioxidants 2022, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Song, M.K.; Adams, L.; Lee, J.H.; Kim, Y.-S. NXP031 prevents dopaminergic neuronal loss and oxidative damage in the AAV-WT-α-synuclein mouse model of Parkinson’s disease. PLoS ONE 2022, 17, e0272085. [Google Scholar] [CrossRef] [PubMed]
- Azodi, S.; Jacobson, S. Cytokine Therapies in Neurological Disease. Neurotherapeutics 2016, 13, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Al-Waili, D.; Hassan, A.; Fan, G.-C.; Xin, M.; Hao, J. Inhibition of cerebral vascular inflammation by brain endothelium-targeted oligodeoxynucleotide complex. Neuroscience 2016, 329, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Cosacak, M.I.; Bhattarai, P.; Reinhardt, S.; Petzold, A.; Dahl, A.; Zhang, Y.; Kizil, C. Single-Cell Transcriptomics Analyses of Neural Stem Cell Heterogeneity and Contextual Plasticity in a Zebrafish Brain Model of Amyloid Toxicity. Cell Rep. 2019, 27, 1307–1318.e3. [Google Scholar] [CrossRef] [Green Version]
- Huff, W.X.; Kwon, J.H.; Henriquez, M.; Fetcko, K.; Dey, M. The Evolving Role of CD8+CD28− Immunosenescent T Cells in Cancer Immunology. Int. J. Mol. Sci. 2019, 20, 2810. [Google Scholar] [CrossRef] [Green Version]
- Salvador, A.F.; de Lima, K.A.; Kipnis, J. Neuromodulation by the immune system: A focus on cytokines. Nat. Rev. Immunol. 2021, 21, 526–541. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, E.K.; Brown, D.R. Senescent Microglia: The Key to the Ageing Brain? Int. J. Mol. Sci. 2021, 22, 4402. [Google Scholar] [CrossRef]
- Vaknine, S.; Soreq, H. Central and peripheral anti-inflammatory effects of acetylcholinesterase inhibitors. Neuropharmacology 2020, 168, 108020. [Google Scholar] [CrossRef]
- Al-Ahmady, Z.S. Selective drug delivery approaches to lesioned brain through blood brain barrier disruption. Expert Opin. Drug Deliv. 2018, 15, 335–349. [Google Scholar] [CrossRef] [Green Version]
- Moni, M.M.R.; Begum, M.M.; Uddin, M.S.; Ashraf, G.M. Deciphering the Role of Nanoparticle-based Treatment for Parkinson’s Disease. Curr. Drug Metab. 2021, 22, 550–560. [Google Scholar] [CrossRef]
- Pluvinage, J.V.; Wyss-Coray, T. Systemic factors as mediators of brain homeostasis, ageing and neurodegeneration. Nat. Rev. Neurosci. 2020, 21, 93–102. [Google Scholar] [CrossRef]
- Krieg, A.M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 2002, 20, 709–760. [Google Scholar] [CrossRef]
- Bruno, J.G. Potential Inherent Stimulation of the Innate Immune System by Nucleic Acid Aptamers and Possible Corrective Approaches. Pharmaceuticals 2018, 11, 62. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; An, M.; Li, M.; Liu, H. Immunostimulatory Properties of Lipid Modified CpG Oligonucleotides. Mol. Pharm. 2017, 14, 2815–2823. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, J.; Krieg, A.M. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv. Drug Deliv. Rev. 2009, 61, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Shukla, D.; Namperumalsamy, P.; Goldbaum, M.; Cunningham, E.T., Jr. Pegaptanib sodium for ocular vascular disease. Indian J. Ophthalmol. 2007, 55, 427–430. [Google Scholar] [CrossRef]
- Tuano, K.S.; Seth, N.; Chinen, J. Secondary immunodeficiencies: An overview. Ann. Allergy Asthma Immunol. 2021, 127, 617–626. [Google Scholar] [CrossRef]
- Fu, Z.; Xiang, J. Aptamer-Functionalized Nanoparticles in Targeted Delivery and Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 9123. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Abellanas, M.A.; Tsitsou-Kampeli, A.; Suzzi, S. The brain-immune ecosystem: Implications for immunotherapy in defeating neurodegenerative diseases. Neuron 2022, 110, 3421–3424. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, A.H.-Y.; Wu, A.J.; Ho, O.K.-Y.; Leung, M.M.-K.; Huang, A.S.; Yu, Y.; Zhang, G.; Lyu, A.; Li, M.; Cheung, K.-H. Exploring the Potential of Aptamers in Targeting Neuroinflammation and Neurodegenerative Disorders: Opportunities and Challenges. Int. J. Mol. Sci. 2023, 24, 11780. https://doi.org/10.3390/ijms241411780
Kong AH-Y, Wu AJ, Ho OK-Y, Leung MM-K, Huang AS, Yu Y, Zhang G, Lyu A, Li M, Cheung K-H. Exploring the Potential of Aptamers in Targeting Neuroinflammation and Neurodegenerative Disorders: Opportunities and Challenges. International Journal of Molecular Sciences. 2023; 24(14):11780. https://doi.org/10.3390/ijms241411780
Chicago/Turabian StyleKong, Anna Hau-Yee, Aston Jiaxi Wu, Olivia Ka-Yi Ho, Maggie Ming-Ki Leung, Alexis Shiying Huang, Yuanyuan Yu, Ge Zhang, Aiping Lyu, Min Li, and King-Ho Cheung. 2023. "Exploring the Potential of Aptamers in Targeting Neuroinflammation and Neurodegenerative Disorders: Opportunities and Challenges" International Journal of Molecular Sciences 24, no. 14: 11780. https://doi.org/10.3390/ijms241411780
APA StyleKong, A. H. -Y., Wu, A. J., Ho, O. K. -Y., Leung, M. M. -K., Huang, A. S., Yu, Y., Zhang, G., Lyu, A., Li, M., & Cheung, K. -H. (2023). Exploring the Potential of Aptamers in Targeting Neuroinflammation and Neurodegenerative Disorders: Opportunities and Challenges. International Journal of Molecular Sciences, 24(14), 11780. https://doi.org/10.3390/ijms241411780