Aloe-Emodin Ameliorates Cecal Ligation and Puncture-Induced Sepsis
Abstract
:1. Introduction
2. Results
2.1. AE Modulated Inflammatory Cytokine Expression on LPS-Stimulated RAW264.7 Cells
2.2. Therapeutic Effect of AE in Mice with CLP-Induced Sepsis
2.3. AE Reduced Inflammatory Cytokine Levels in Mice with CLP-Induced Sepsis
2.4. AE Ameliorated CLP-Induced Pathology
2.5. AE Altered the Hematology Parameters in Mice with CLP-Induced Sepsis
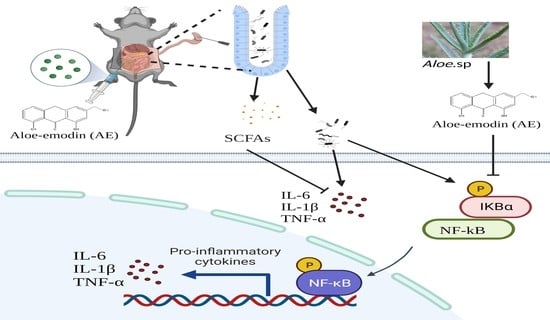
2.6. AE Regulated Intestinal Flora Homeostasis in Mice with CLP-Induced Sepsis
2.7. AE Facilitated the Production of SCFAs in Mice Afflicted with CLP-Induced Sepsis
2.8. AE Inhibited the CLP-Induced Inflammatory Response
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Lines and Culture Conditions
4.3. Cell Viability Assay
4.4. Animals
4.5. CLP Model of Sepsis
4.6. Experimental Protocol
4.7. Quantitative Reverse Transcription PCR (RT-qPCR)
4.8. ELISA
4.9. Western Blotting
4.10. Histopathological Examination
4.11. Hematological Parameters
4.12. Immunohistochemical Assay
4.13. DNA Extraction and 16S rRNA Sequencing
4.14. Detection of SCFAs
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [PubMed]
- Tom, P.D.; Manu, S.; Joost, W.W. The immunology of sepsis. Immunity 2021, 54, 2450–2464. [Google Scholar]
- Vincent, J.L.; Marshall, J.C.; Namendys-Silva, S.A.; François, B.; Martin-Loeches, I.; Lipman, J.; Reinhart, K.; Antonelli, M.; Pickkers, P.; Njimi, H.; et al. Assessment of the worldwide burden of critical illness: The intensive care over nations (ICON) audit. Lancet Respir. Med. 2014, 2, 380–386. [Google Scholar] [CrossRef]
- Rocheteau, P.; Chatre, L.; Briand, D.; Mebarki, M.; Jouvion, G.; Bardon, J.; Crochemore, C.; Serrani, P.; Lecci, P.P.; Latil, M.; et al. Sepsis induces long-term metabolic and mitochondrial muscle stem cell dysfunction amenable by mesenchymal stem cell therapy. Nat. Commun. 2015, 6, 10145. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.; Cai, S.; Su, J. The pathogenesis of sepsis and potential therapeutic targets. Int. J. Mol. Sci. 2019, 20, 5376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotchkiss, R.S.; Sherwood, E.R. Getting sepsis therapy right. Science 2015, 347, 1201–1202. [Google Scholar] [CrossRef] [Green Version]
- Korneev, K.V. Mouse models of sepsis and septic shock. Mol. Biol. (Mosk) 2019, 53, 799–814. [Google Scholar] [CrossRef]
- Fernández, C.; Palazuelos, C.; Cristobal Poch, L.; Gomez Ruiz, M. A probabilistic model for the prediction of intra-abdominal infection after colorectal surgery. Int. J. Colorectal Dis. 2021, 36, 2481–2488. [Google Scholar] [CrossRef]
- Kullberg, R.F.J.; Wiersinga, W.J.; Haak, B.W. Gut microbiota and sepsis: From pathogenesis to novel treatments. Curr. Opin. Gastroenterol. 2021, 37, 578–585. [Google Scholar] [CrossRef]
- Sonawane, S.K.; Gokhale, J.S.; Mulla, M.Z.; Kandu, V.R.; Patil, S. A comprehensive overview of functional and rheological properties of aloe vera and its application in foods. J. Food Sci. Technol. 2021, 58, 1217–1226. [Google Scholar] [CrossRef]
- Kumar, S.; Yadav, M.; Yadav, A.; Rohilla, P.; Yadav, J.P. Antiplasmodial potential and quantification of aloin and aloe-emodin in Aloe vera collected from different climatic regions of India. BMC Complement. Altern. Med. 2017, 17, 369. [Google Scholar]
- Dong, X.; Zeng, Y.; Liu, Y.; You, L.; Yin, X.; Fu, J.; Ni, J. Aloe-emodin: A review of its pharmacology, toxicity, and pharmacokinetics. Phytother. Res. 2020, 34, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU), 2021/468 of 18 March 2021 Amending Annex III to Regulation (EC) No 1925/2006 of the European Parliament and of the Council as Regards Botanical Species Containing Hydroxyanthracene Derivatives. Available online: https://eur-lex.europa.eu/eli/reg/2021/468/oj (accessed on 18 July 2023).
- Galli, C.L.; Cinelli, S.; Ciliutti, P.; Melzi, G.; Marinovich, M. Aloe-emodin, a hydroxyanthracene derivative, is not genotoxic in an in vivo comet test. Regul. Toxicol. Pharmacol. 2021, 124, 104967. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Cao, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W.; Xie, Y. Transformation and degradation of barbaloin in aqueous solutions and aloe powder under different processing conditions. Food Biosci. 2021, 43, 101279. [Google Scholar] [CrossRef]
- Friedman, M.; Xu, A.; Lee, R.; Nguyen, D.N.; Phan, T.A.; Hamada, S.M.; Panchel, R.; Tam, C.C.; Kim, J.H.; Cheng, L.W.; et al. The inhibitory activity of anthraquinones against pathogenic protozoa, bacteria, and fungi and the relationship to structure. Molecules 2020, 25, 3101. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, H.; Meng, X.; Wang, F.; Wang, P. Aloe-emodin from rhubarb (Rheum rhabarbarum) inhibits lipopolysaccharide-induced inflammatory responses in RAW264.7 macrophages. J. Ethnopharmacol. 2014, 153, 846–853. [Google Scholar] [CrossRef]
- Gao, H.; Ren, Y.; Liu, C. Aloe-emodin suppresses oxidative stress and inflammation via a PI3K-dependent mechanism in a murine model of sepsis. Evid. Based Complement. Alternat. Med. 2022, 2022, 9697887. [Google Scholar] [CrossRef]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef]
- Silva, S.C.D.; Baggio-Zappia, G.L.; Brunialti, M.K.C.; Assunçao, M.S.C.; Azevedo, L.C.P.; Machado, F.R.; Salomao, R. Evaluation of Toll-like, chemokine, and integrin receptors on monocytes and neutrophils from peripheral blood of septic patients and their correlation with clinical outcomes. Braz. J. Med. Biol. Res. 2014, 47, 384–393. [Google Scholar] [CrossRef] [Green Version]
- Weber, G.F.; Chousterman, B.G.; He, S.; Fenn, A.M.; Nairz, M.; Anzai, A.; Brenner, T.; Uhle, F.; Iwamoto, Y.; Robbins, C.S.; et al. Interleukin-3 amplifies acute inflammation and is a potential therapeutic target in sepsis. Science 2015, 347, 1260–1265. [Google Scholar] [CrossRef] [Green Version]
- Karbian, N.; Abutbul, A.; El-Amore, R.; Eliaz, R.; Beeri, R.; Reicher, B.; Mevorach, D. Apoptotic cell therapy for cytokine storm associated with acute severe sepsis. Cell Death Dis. 2020, 11, 535. [Google Scholar] [CrossRef]
- Arosio, B.; Gagliano, N.; Fusaro, L.M.; Parmeggiani, L.; Tagliabue, J.; Galetti, P.; De Castri, D.; Moscheni, C.; Annoni, G. Aloe-Emodin quinone pretreatment reduces acute liver injury induced by carbon tetrachloride. Pharmacol. Toxicol. 2000, 87, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Geng, S.; Wang, X.; Cui, Z.; Ma, W.; Yuan, M.; Liu, C.; Ji, Y. Aloe-emodin-mediated antimicrobial photodynamic therapy against multidrug-resistant Acinetobacter baumannii: An in vivo study. Photodiagnosis Photodyn. Ther. 2021, 34, 102311. [Google Scholar] [PubMed]
- Lin, C.F.; Chuang, S.Y.; Huang, T.H.; Nguyen, T.M.H.; Wang, P.W.; Alalaiwe, A.; Fang, J.Y. A systematic comparison of the effect of topically applied anthraquinone aglycones to relieve psoriasiform lesions: The evaluation of percutaneous absorption and anti-inflammatory potency. Biomed. Pharmacother. 2022, 145, 112482. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Ding, S.; Mao, Z.; You, L.; Ruan, Y. Integrated analysis of potential pathways by which aloe-emodin induces the apoptosis of colon cancer cells. Cancer Cell Int. 2021, 21, 238. [Google Scholar] [CrossRef]
- Shang, H.; Guo, J.; Wang, P.; Li, L.; Tian, Y.; Li, X.; Zou, Z. Design, synthesis and anti-inflammatory evaluation of aloe-emodin derivatives as potential modulators of Akt, NF-κB and JNK signaling pathways. Eur. J. Med. Chem. 2022, 238, 114511. [Google Scholar] [CrossRef] [PubMed]
- Klingensmith, N.J.; Coopersmith, C.M. The gut as the motor of multiple organ dysfunction in critical illness. Crit. Care Clin. 2016, 32, 203–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haak, B.W.; Wiersinga, W.J. The role of the gut microbiota in sepsis. Lancet Gastroenterol. Hepatol. 2017, 2, 135–143. [Google Scholar] [CrossRef]
- Bae, M.; Cassilly, C.D.; Liu, X.; Park, S.M.; Tusi, B.K.; Chen, X.; Kwon, J.; Filipčík, P.; Bolze, A.S.; Liu, Z.; et al. Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature 2022, 608, 168–173. [Google Scholar]
- Cui, H.; Cai, Y.; Wang, L.; Jia, B.; Li, J.; Zhao, S.; Chu, X.; Lin, J.; Zhang, X.; Bian, Y.; et al. Berberine regulates Treg/Th17 balance to treat ulcerative colitis through modulating the gut microbiota in the colon. Front. Pharmacol. 2018, 9, 571. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Cheng, N.; Zhou, W.; Chen, S.; Wang, Q.; Gao, H.; Xue, X.; Wu, L.; Cao, W. Honey polyphenols ameliorate DSS-induced ulcerative colitis via modulating gut microbiota in rats. Mol. Nutr. Food Res. 2019, 63, e1900638. [Google Scholar] [PubMed]
- Liu, Y.; Zhou, M.; Yang, M.; Jin, C.; Song, Y.; Chen, J.; Gao, M.; Ai, Z.; Su, D. Pulsatilla chinensis saponins ameliorate inflammation and DSS-induced ulcerative colitis in rats by regulating the composition and diversity of intestinal flora. Front. Cell. Infect. Microbiol. 2021, 11, 728929. [Google Scholar] [CrossRef]
- Kim, C.H. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell. Mol. Immunol. 2021, 18, 1161–1171. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Su, J.; Guo, K.; Huang, M.; Liu, Y.; Zhang, J.; Sun, L.; Li, D.; Pang, K.; Wang, G.; Chen, L.; et al. Fucoxanthin, a marine xanthophyll isolated from Conticribra weissflogii ND-8: Preventive anti-inflammatory effect in a mouse model of sepsis. Front. Pharmacol. 2019, 10, 906. [Google Scholar] [CrossRef] [Green Version]
- Alverdy, J.C.; Keskey, R.; Thewissen, R. Can the Cecal Ligation and Puncture model be repurposed to better inform therapy in human sepsis? Infect. Immun. 2020, 88, e00942-19. [Google Scholar] [CrossRef]
- Shrum, B.; Anantha, R.V.; Xu, S.X.; Donnelly, M.; Haeryfar, S.M.; McCormick, J.K.; Mele, T. A robust scoring system to evaluate sepsis severity in an animal model. Notes. BMC Res. Notes 2014, 7, 233. [Google Scholar]
- Wang, P.; Feng, Z.; Sang, X.; Chen, W.; Zhang, X.; Xiao, J.; Chen, Y.; Chen, Q.; Yang, M.; Su, J. Kombucha ameliorates LPS-induced sepsis in a mouse model. Food Funct. 2021, 12, 10263–10280. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Li, Y.; Chen, C.; Yin, T.; Fang, Q.; Zhao, Y.; Lv, W.; Liu, Z.; Chen, Y.; Shen, L. Caveolin-1 identified as a key mediator of acute lung injury using bioinformatics and functional research. Cell Death Dis. 2022, 13, 686. [Google Scholar] [CrossRef]
- BMK Cloud Platform. Available online: https://www.biocloud.net.do (accessed on 10 March 2023).
- Gu, H.; Jasbi, P.; Patterson, J.; Jin, Y. Enhanced detection of short-chain fatty acids using gas chromatography mass spectrometry. Curr. Protoc. 2021, 1, e177. [Google Scholar]











| Antibody | Source | Identifier |
|---|---|---|
| NF-kB p65 | ABclonal | A19653 |
| Phospho-NF-kB p65 | ABclonal | AP0124 |
| GAPDH | ABclonal | A19056 |
| IκBα | ABclonal | A1187 |
| Phospho-IκBα | ABclonal | AP0999 |
| Name | Sequence (5′–3′) |
|---|---|
| IL-6 | F: TACCACTTCACAAGTCGGAGGC |
| R: CTGCAAGTGCATCATCGTTGTTC | |
| IL-1β | F: TGGGAAACAACAGTGGTCAGG |
| R: CCATCAGAGGCAAGGAGGAA | |
| TNF-α | F: GAGTGACAAGCCTGTAGCC |
| R: CTCCTGGTATGAGATAGCAAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.; Chen, S.; Xiao, J.; Feng, Z.; Hu, S.; Su, Q.; Chen, Q.; Chen, D. Aloe-Emodin Ameliorates Cecal Ligation and Puncture-Induced Sepsis. Int. J. Mol. Sci. 2023, 24, 11972. https://doi.org/10.3390/ijms241511972
Su J, Chen S, Xiao J, Feng Z, Hu S, Su Q, Chen Q, Chen D. Aloe-Emodin Ameliorates Cecal Ligation and Puncture-Induced Sepsis. International Journal of Molecular Sciences. 2023; 24(15):11972. https://doi.org/10.3390/ijms241511972
Chicago/Turabian StyleSu, Jingqian, Siyuan Chen, Jianbin Xiao, Zhihua Feng, Shan Hu, Qiaofen Su, Qi Chen, and Duo Chen. 2023. "Aloe-Emodin Ameliorates Cecal Ligation and Puncture-Induced Sepsis" International Journal of Molecular Sciences 24, no. 15: 11972. https://doi.org/10.3390/ijms241511972
APA StyleSu, J., Chen, S., Xiao, J., Feng, Z., Hu, S., Su, Q., Chen, Q., & Chen, D. (2023). Aloe-Emodin Ameliorates Cecal Ligation and Puncture-Induced Sepsis. International Journal of Molecular Sciences, 24(15), 11972. https://doi.org/10.3390/ijms241511972