Resveratrol-Loaded Attalea funifera Oil Organogel Nanoparticles: A Potential Nanocarrier against A375 Human Melanoma Cells
Abstract
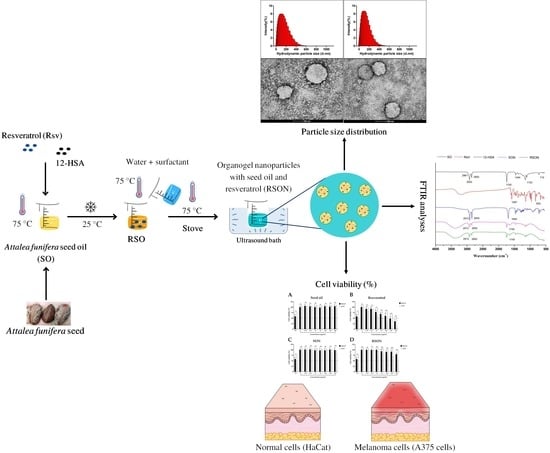
:1. Introduction
2. Results and Discussion
2.1. Attalea funifera Seed Oil Characterization
2.2. Organogel Characterization
2.2.1. Particle Size and Polydispersity Index
2.2.2. pH Analysis and Electrical Conductivity
2.2.3. Resveratrol Entrapment Efficiency in Organogel Nanoparticles
2.2.4. Fourier-Transform Infrared Spectroscopy Analysis
2.2.5. Organogel Nanoparticle Morphology Analysis by Transmission Electron Microscopy and Size Distribution by Dynamic Light Scattering
2.3. Cell Viability Assay for Organogel Nanoparticles and Their Compounds
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material and Seed Oil Extraction
3.3. Physicochemical Characterization and Fatty Acid Composition of the Seed Oil
3.4. Organogel Preparation and Characterization
3.5. Organogel Nanoparticles Preparation and Characterization
3.5.1. Organogel Nanoparticles Preparation
3.5.2. Organogel Nanoparticles Size Distribution and Zeta Potential
3.5.3. Organogel Nanoparticles pH Analysis and Electrical Conductivity
3.5.4. Resveratrol-Entrapment Efficiency of Organogel Nanoparticles
3.5.5. Morphological Study by Transmission Electron Microscopy (TEM)
3.6. Cell Viability Assay of Organogel Nanoparticles and Their Compounds
3.7. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmadi, R.; Ebrahimzadeh, M.A. Resveratrol—A comprehensive review of recent advances in anticancer drug design and development. Eur. J. Med. Chem. 2020, 200, 112356. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.; Bannister, P.; Rogers, I.; Sundin, J.; Al-Ayadhy, B.; James, P.; Mcnally, R. Changing epidemiology and age-specific incidence of cutaneous malignant melanoma in England: An analysis of the national cancer registration data by age, gender and anatomical site, 1981–2018. Lancet Reg. Health Eur. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Gong, C.; Xia, H. Resveratrol suppresses melanoma growth by promoting autophagy through inhibiting the PI3K/AKT/mTOR signaling pathway. Exp. Ther. Med. 2020, 19, 1878–1886. [Google Scholar] [CrossRef]
- Santos, A.C.; Pereira, I.; Pereira-Silva, M.; Ferreira, L.; Caldas, M.; Magalhães, M.; Figueiras, A.; Ribeiro, A.J.; Veiga, F. Nanocarriers for resveratrol delivery: Impact on stability and solubility concerns. Trends Food Sci. Technol. 2019, 91, 483–497. [Google Scholar] [CrossRef]
- Szulc-Musioł, B.; Sarecka-Hujar, B. The Use of Micro- and Nanocarriers for Resveratrol Delivery into and across the Skin in Different Skin Diseases—A Literature Review. Pharmaceutics 2021, 13, 451. [Google Scholar] [CrossRef] [PubMed]
- Palliyage, G.H.; Hussein, N.; Mimlitz, M.; Weeder, C.; Alnasser, M.H.A.; Singh, S.; Ekpenyong, A.; Tiwari, A.K.; Chauhan, H. Novel Curcumin-Resveratrol Solid Nanoparticles Synergistically Inhibit Proliferation of Melanoma Cells. Pharm. Res. 2021, 38, 851–871. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Potential of Nanoparticles as Permeation Enhancers and Targeted Delivery Options for Skin: Advantages and Disadvantages. Drug Des. Devel. Ther. 2020, 14, 3271–3289. [Google Scholar] [CrossRef]
- Yi, G.; Son, J.; Yoo, J.; Park, C.; Koo, H. Emulsan-based nanoparticles for in vivo drug delivery to tumors. Biochem. Biophys. Res. Commun. 2019, 508, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Suita, Y.; Miriyala, S.; Dean, J.; Tapinos, N.; Shen, J. Advances in Lipid-Based Nanoparticles for Cancer Chemoimmunotherapy. Pharmaceutics 2021, 13, 520. [Google Scholar] [CrossRef]
- Martin, B.; Brouillet, F.; Franceschi, S.; Perez, E. Evaluation of Organogel Nanoparticles as Drug Delivery System for Lipophilic Compounds. AAPS PharmSciTech 2017, 18, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 319. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ahmad, U.; Akhtar, J.; Badruddeen; Khan, M.M. Engineered nano scale formulation strategies to augment efficiency of nutraceuticals. J. Funct. Foods 2019, 62, 103554. [Google Scholar] [CrossRef]
- Ting, Y.; Jiang, Y.; Ho, C.-T.; Huang, Q. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J. Funct. Foods 2014, 7, 112–128. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Luo, L.-J.; Yang, C.-J.; Lai, J.-Y. Highly Retina-Permeating and Long-Acting Resveratrol/Metformin Nanotherapeutics for Enhanced Treatment of Macular Degeneration. ACS Nano 2023, 17, 168–183. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Yang, H.; Liu, X.; Lu, Z. Preparation and biological activity studies of resveratrol loaded ionically cross-linked chitosan-TPP nanoparticles. Carbohydr. Polym. 2017, 175, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, Y.; Su, Y.; Li, S.; Dong, J.; He, Q.; Cao, Y.; Lu, T.; Qin, L. Resveratrol-Loaded Solid Lipid Nanoparticle Supplementation Ameliorates Physical Fatigue by Improving Mitochondrial Quality Control. Crystals 2019, 9, 559. [Google Scholar] [CrossRef] [Green Version]
- Neves, A.; Martins, S.; Segundo, M.; Reis, S. Nanoscale Delivery of Resveratrol towards Enhancement of Supplements and Nutraceuticals. Nutrients 2016, 8, 131. [Google Scholar] [CrossRef] [Green Version]
- Venturini, C.G.; Bruinsmann, F.A.; Contri, R.V.; Fonseca, F.N.; Frank, L.A.; D’Amore, C.M.; Raffin, R.P.; Buffon, A.; Pohlmann, A.R.; Guterres, S.S. Co-encapsulation of imiquimod and copaiba oil in novel nanostructured systems: Promising formulations against skin carcinoma. Eur. J. Pharm. Sci. 2015, 79, 36–43. [Google Scholar] [CrossRef]
- Bahreyni, A.; Mohamud, Y.; Luo, H. Recent advancements in immunotherapy of melanoma using nanotechnology-based strategies. Biomed. Pharmacother. 2023, 159, 114243. [Google Scholar] [CrossRef]
- Kumar, S.; Dilbaghi, N.; Rani, R.; Bhanjana, G. Nanotechnology as Emerging Tool for Enhancing Solubility of Poorly Water-Soluble Drugs. Bionanoscience 2012, 2, 227–250. [Google Scholar] [CrossRef]
- Kirilov, P. Colloidal Dispersions of Gelled Lipid Nanoparticles (GLN): Concept and Potential Applications. Gels 2017, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Dourado, D.; de Oliveira, M.C.; de Araujo, G.R.S.; Amaral-Machado, L.; Porto, D.L.; Aragão, C.F.S.; do Nascimento Alencar, E.; do Egito, E.S.T. Low-surfactant microemulsion, a smart strategy intended for curcumin oral delivery. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129720. [Google Scholar] [CrossRef]
- Esposito, C.L.; Kirilov, P.; Roullin, V.G. Organogels, promising drug delivery systems: An update of state-of-the-art and recent applications. J. Control. Release 2018, 271, 1–20. [Google Scholar] [CrossRef]
- Hasegawa, T.; Umemura, J.; Takenaka, T. Fourier transform infrared metal overlayer attenuated total reflection spectra of Langmuir-Blodgett films of 12-hydroxystearic acid and its cadmium salt. Thin Solid Film. 1992, 210–211, 583–585. [Google Scholar] [CrossRef]
- Nouri, V.; Siqueira-Moura, M.; Payre, B.; De Almeida, O.; Déjugnat, C.; Franceschi, S.; Perez, E. How an organogelator can gelate water: Gelation transfer from oil to water induced by a nanoemulsion. Soft Matter 2020, 16, 2371–2378. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, J.; Cowley, A.; Preez, J.; Gerber, M.; Du Plessis, J. Penetration enhancing effects of selected natural oils utilized in topical dosage forms. Drug Dev. Ind. Pharm. 2015, 41, 2045–2054. [Google Scholar] [CrossRef]
- Efendy Goon, D.; Sheikh Abdul Kadir, S.H.; Latip, N.A.; Rahim, S.A.; Mazlan, M. Palm Oil in Lipid-Based Formulations and Drug Delivery Systems. Biomolecules 2019, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Fischer, C.L.; Blanchette, D.R.; Brogden, K.A.; Dawson, D.V.; Drake, D.R.; Hill, J.R.; Wertz, P.W. The roles of cutaneous lipids in host defense. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2014, 1841, 319–322. [Google Scholar] [CrossRef] [Green Version]
- Ghani, N.; Channip, A.-A.; Hwa, P.; Ja’afar, F.; Yasin, H.; Usman, A. Physicochemical properties, antioxidant capacities, and metal contents of virgin coconut oil produced by wet and dry processes. Food Sci. Nutr. 2018, 6, 1298–1306. [Google Scholar] [CrossRef]
- Narayanan, A.; Ananda Baskaran, S.; Amalaradjou, M.; Venkitanarayanan, K. Anticarcinogenic Properties of Medium Chain Fatty Acids on Human Colorectal, Skin and Breast Cancer Cells in Vitro. Int. J. Mol. Sci. 2015, 16, 5014–5027. [Google Scholar] [CrossRef] [Green Version]
- Oliveira Filho, G.C.; Sousa Mota, R.C.; Conceição, A.C.R.; Leão, M.A.; Araujo Filho, O.O. Data generated by the hybridization of mechanical properties of composite reinforced by piassava fiber fabric. Data Br. 2018, 21, 2330–2338. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.A.A.; da Silva, J.W.; dos Santos, S.M.; Rodrigues, M.d.F.; Silva, C.J.A.; da Silva, M.V.; Correia, M.T.S.; Albuquerque, J.F.C.; Melo, C.M.L.; Silva, T.G.; et al. In Vitro and In Vivo Wound Healing and Anti-Inflammatory Activities of Babassu Oil (Attalea speciosa Mart. Ex Spreng., Arecaceae). Evid.-Based Complement. Altern. Med. 2020, 2020, 8858291. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission. Codex Standard for Named Vegetable Oils (Codex-Stan 210-1999), Amended 2005, 2011, 2013, 2015. FAO/WHO, Rome, 2013. pp. 1–13. Available online: https://inspection.canada.ca/DAM/DAM-food-aliments/WORKAREA/DAM-food-aliments/text-texte/codex_food_stand_named_veg_oils_1532975057193_eng.pdf (accessed on 25 July 2023).
- da Silva, M.J.F.; Rodrigues, A.M.; Vieira, I.R.S.; Neves, G.d.A.; Menezes, R.R.; Graça do Rosário Gonçalves, E.; Pires, M.C.C. Development and characterization of a babassu nut oil-based moisturizing cosmetic emulsion with a high sun protection factor. RSC Adv. 2020, 10, 26268–26276. [Google Scholar] [CrossRef]
- Tiwari, N.; Sonzogni, A.S.; Calderón, M. Can dermal delivery of therapeutics be improved using thermoresponsive nanogels? Nanomedicine 2019, 14, 2891–2895. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasany, S.; Mozafari, M. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Bolkestein, M.; de Blois, E.; Koelewijn, S.J.; Eggermont, A.M.M.; Grosveld, F.; de Jong, M.; Koning, G.A. Investigation of Factors Determining the Enhanced Permeability and Retention Effect in Subcutaneous Xenografts. J. Nucl. Med. 2016, 57, 601–607. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, S.; Rietjens, I.; Singh, M.; Atkins, T.; Purkait, T.; Xu, Z.; Regli, S.; Shukaliak, A.; Clark, R.; Mitchell, B.; et al. Cytotoxicity of Surface-functionalized Silicon and Germanium Nanoparticles: The Dominant Role of Surface Charges. Nanoscale 2013, 5, 4870–4883. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Missana, T.; Adell, A. On the Applicability of DLVO Theory to the Prediction of Clay Colloids Stability. J. Colloid Interface Sci. 2000, 230, 150–156. [Google Scholar] [CrossRef]
- Kerwin, B.A. Polysorbates 20 and 80 Used in the Formulation of Protein Biotherapeutics: Structure and Degradation Pathways. J. Pharm. Sci. 2008, 97, 2924–2935. [Google Scholar] [CrossRef] [PubMed]
- Kishore, R.S.K.; Pappenberger, A.; Dauphin, I.B.; Ross, A.; Buergi, B.; Staempfli, A.; Mahler, H.-C. Degradation of Polysorbates 20 and 80: Studies on Thermal Autoxidation and Hydrolysis. J. Pharm. Sci. 2011, 100, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Amagai, M. Dissecting the formation, structure and barrier function of the stratum corneum. Int. Immunol. 2015, 27, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Kamaruzaman, N.; Yusop, S.M. Determination of stability of cosmetic formulations incorporated with water-soluble elastin isolated from poultry. J. King Saud Univ.-Sci. 2021, 33, 101519. [Google Scholar] [CrossRef]
- Fürtjes, T.; Weiss, K.T.; Filbry, A.; Rippke, F.; Schreml, S. Impact of a pH 5 Oil-in-Water Emulsion on Skin Surface pH. Ski. Pharmacol. Physiol. 2017, 30, 292–297. [Google Scholar] [CrossRef]
- Musa, S.H.; Basri, M.; Fard Masoumi, H.R.; Shamsudin, N.; Salim, N. Enhancement of physicochemical properties of nanocolloidal carrier loaded with cyclosporine for topical treatment of psoriasis: In vitro diffusion and in vivo hydrating action. Int. J. Nanomed. 2017, 12, 2427–2441. [Google Scholar] [CrossRef] [Green Version]
- Lôbo de Souza, M.; Dourado, D.; Pinheiro Lôbo, I.; Couto Pires, V.; Nunes de Oliveira Araújo, S.; de Souza Rebouças, J.; Costa, A.M.; Pinho Fernandes, C.; Machado Tavares, N.; de Paula Pereira, N.; et al. Impact of a pH 5 Oil-in-Water Emulsion on Skin Surface pH. J. King Saud Univ. Sci. 2017, 30, 337–351. [Google Scholar]
- Badea, G.; Lacatusu, I.; Ott, C.; Badea, N.; Grafu, I.; Meghea, A. Integrative approach in prevention and therapy of basal cellular carcinoma by association of three actives loaded into lipid nanocarriers. J. Photochem. Photobiol. B. 2015, 147, 1–8. [Google Scholar] [CrossRef]
- Gandhi, K.; Sharma, R.; Seth, R.; Mann, B. Detection of coconut oil in ghee using ATR-FTIR and chemometrics. Appl. Food Res. 2022, 2, 100035. [Google Scholar] [CrossRef]
- Koca, N.; Kocaoglu-Vurma, N.A.; Harper, W.J.; Rodriguez-Saona, L.E. Application of temperature-controlled attenuated total reflectance-mid-infrared (ATR-MIR) spectroscopy for rapid estimation of butter adulteration. Food Chem. 2010, 121, 778–782. [Google Scholar] [CrossRef]
- Sari, F.; Susanto, B.H.; Bismo, S. The potential utilization of coconut oil and palm oil as raw material of alkanolamide under alkaline conditions. IOP Conf. Ser. Earth Environ. Sci. 2018, 105, 12035. [Google Scholar] [CrossRef]
- Tan, K.-X.; Ng, L.-L.E.; Loo, S.C.J. Formulation Development of a Food-Graded Curcumin-Loaded Medium Chain Triglycerides-Encapsulated Kappa Carrageenan (CUR-MCT-KC) Gel Bead Based Oral Delivery Formulation. Materials 2021, 14, 2783. [Google Scholar] [CrossRef]
- Elham, A.; Rostamabadi, H.; Jafari, S.M. Chapter One—Characterization of Nanoencapsulated Food Ingredients. In Nanoencapsulation in the Food Industry, Characterization of Nanoencapsulated Food Ingredients; Jafari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–50. ISBN 9780128156674. [Google Scholar] [CrossRef]
- Chen, C.; Chen, C.; Li, Y.; Gu, R.; Yan, X. Characterization of lipid-based nanomedicines at the single-particle level. Fundam. Res. 2023, 3, 488–504. [Google Scholar] [CrossRef]
- Siqueira-Moura, M.P.; Franceschi-Messant, S.; Blanzat, M.; Ré, M.I.; Perez, E.; Rico-Lattes, I.; Lattes, A.; Tedesco, A.C. Gelled oil particles: A new approach to encapsulate a hydrophobic metallophthalocyanine. J. Colloid Interface Sci. 2013, 401, 155–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaneva, Z.; Georgieva, N. Chapter 5—Physicochemical and morphological characterization of pharmaceutical nanocarriers and mathematical modeling of drug encapsulation/release mass transfer processes. In Nanoscale Fabrication, Optimization, Scale-Up and Biological Aspects of Pharmaceutical Nanotechnology; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 173–218. ISBN 978-0-12-813629-4. [Google Scholar]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lôbo de Souza, M.; Dourado, D.; Pinheiro Lôbo, I.; Couto Pires, V.; Nunes de Oliveira Araújo, S.; de Souza Rebouças, J.; Costa, A.M.; Pinho Fernandes, C.; Machado Tavares, N.; de Paula Pereira, N.; et al. Wild Passiflora (Passiflora spp.) seed oils and their nanoemulsions induce proliferation in HaCaT keratinocytes cells. J. Drug Deliv. Sci. Technol. 2022, 67, 102803. [Google Scholar] [CrossRef]
- ISO-10993-5; Part 5—Tests for In Vitro Cytotoxicity (ISO 10993-5:2009). Biological Evaluation of Medical Devices. 3rd ed. International Organization for Standardization: London, UK, 2009; pp. 1–34. Available online: https://nhiso.com/wp-content/uploads/2018/05/ISO-10993-5-2009.pdf (accessed on 25 July 2023).
- Lee, J.-H.; Kim, J.-S.; Park, S.-Y.; Lee, Y.-J. Resveratrol induces human keratinocyte damage via the activation of class III histone deacetylase, Sirt1. Oncol. Rep. 2016, 35, 524–529. [Google Scholar] [CrossRef] [Green Version]
- Shaito, A.; Maria, P.; Younis, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.; Eid, A.; Nasrallah, G.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.R.; Nabavi, S.F.; Manayi, A.; Daglia, M.; Hajheydari, Z.; Nabavi, S.M. Resveratrol and the mitochondria: From triggering the intrinsic apoptotic pathway to inducing mitochondrial biogenesis, a mechanistic view. Biochim. Biophys. Acta-Gen. Subj. 2016, 1860, 727–745. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Li, W.; Jiang, X.; Ji, Y.; Wu, X.; Xu, L.; Qiu, Y.; Zhao, K.; Wei, T.; et al. Selective Targeting of Gold Nanorods at the Mitochondria of Cancer Cells: Implications for Cancer Therapy. Nano Lett. 2011, 11, 772–780. [Google Scholar] [CrossRef]
- Govaerts, R. World Checklist of Selected Plant Families. In Catalogue of Life Checklist (August 2017); Bánki, O., Roskov, Y., Vandepitte, L., DeWalt, R.E., Remsen, D., Schalk, P., Orrell, T., Keping, M., Miller, J., Aalbu, R., et al., Eds.; The Catalogue of Life Partnership: Leiden, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Firestone, D. Official Methods and Recommended Practices of the AOCS, 6th ed.; AOCS: Urbana, IL, USA, 2011; ISBN 9781893997745. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, I.A.; Marques, S.S.I.; Cabanelas, I.T.D.; Pereira, S.A.; Druzian, J.I.; de Souza, C.O.; Vich, D.V.; de Carvalho, G.C.; Nascimento, M.A. Screening Microalgae Strains for Biodiesel Production: Lipid Productivity and Estimation of Fuel Quality Based on Fatty Acids Profiles as Selective Criteria. BioEnergy Res. 2013, 6, 1–13. [Google Scholar] [CrossRef]
- Sagiri, S.S.; Singh, V.K.; Pal, K.; Banerjee, I.; Basak, P. Stearic acid based oleogels: A study on the molecular, thermal and mechanical properties. Mater. Sci. Eng. C 2015, 48, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.; Patil, V.; Sagiri, S.S.; Pal, K.; Ray, S.S. Span-60-based organogels as probable matrices for transdermal/topical delivery systems. J. Appl. Polym. Sci. 2012, 125, 852–863. [Google Scholar] [CrossRef]
- Kirilov, P.; Lukyanova, L.; Franceschi-Messant, S.; Perier, V.; Perez, E.; Rico-Lattes, I. A new type of colloidal dispersions based on nanoparticles of gelled oil. Colloids Surf. A Physicochem. Eng. Asp. 2008, 328, 1–7. [Google Scholar] [CrossRef]
- Page, B.; Page, M.; Noel, C. A new fluorometric assay for cytotoxicity measurements in-vitro. Int. J. Oncol. 1993, 3, 473–476. [Google Scholar] [CrossRef]
- De Grandis, R.A.; de Camargo, M.S.; da Silva, M.M.; Lopes, É.O.; Padilha, E.C.; Resende, F.A.; Peccinini, R.G.; Pavan, F.R.; Desideri, A.; Batista, A.A.; et al. Human topoisomerase inhibition and DNA/BSA binding of Ru(II)–SCAR complexes as potential anticancer candidates for oral application. BioMetals 2017, 30, 321–334. [Google Scholar] [CrossRef]



| Compound | Value (%) |
|---|---|
| Caprylic acid (C8:0) | 1.96 ± 0.07 |
| Capric acid (C10:0) | 8.32 ± 0.30 |
| Lauric acid (C12:0) | 55.29 ± 1.93 |
| Myristic acid (C14:0) | 15.13 ± 0.42 |
| Palmitic acid (C16:0) | 6.17 ± 0.47 |
| Stearic acid (C18:0) | 3.26 ± 0.04 |
| Oleic acid (C18:1 ∆9 cis) ω-9 | 8.21 ± 0.22 |
| Linoleic acid (C18:2 ∆9.12 cis) ω-6 | 1.64 ± 0.04 |
| Total saturated fatty acid | 90.13 |
| Total monounsaturated fatty acid | 8.21 |
| Total polyunsaturated fatty acid | 1.64 |
| Time (Day) | SON | RSON | ||
|---|---|---|---|---|
| Particle Size (nm) | Polydispersity Index | Particle Size (nm) | Polydispersity Index | |
| 01 | 86.09 ± 0.24 aA | 0.273 ± 0.004 aA | 76.19 ± 0.93 aB | 0.278 ± 0.002 aA |
| 15 | 84.93 ± 0.35 aA | 0.271 ± 0.004 aB | 76.42 ± 0.34 aB | 0.282 ± 0.004 aA |
| 30 | 85.63 ± 0.33 aA | 0.266 ± 0.009 aA | 75.72 ± 0.65 aB | 0.264 ± 0.004 bA |
| 60 | 85.59 ± 0.30 aA | 0.268 ± 0.006 aA | 75.91 ± 0.09 aB | 0.262 ± 0.002 bA |
| 90 | 85.81 ± 0.59 aA | 0.272 ± 0.008 aA | 75.92 ± 0.04 aB | 0.266 ± 0.002 bA |
| Time (Day) | Zeta Potential (mV) * | |
|---|---|---|
| SON | RSON | |
| 01 | −26.27 ± 1.00 aA | −21.90 ± 0.35 aB |
| 15 | −23.50 ± 0.44 bA | −23.63 ± 0.58 aA |
| 30 | −21.13 ± 0.06 bA | −23.60 ± 0.80 aA |
| 60 | −23.94 ± 0.06 bA | −23.53 ± 1.19 aA |
| 90 | −31.57 ± 1.76 bA | −32.27 ± 1.00 bA |
| Time (Day) | pH * | |
|---|---|---|
| SON | RSON | |
| 01 | 6.03 ± 0.07 aA | 5.99 ± 0.04 aA |
| 15 | 6.19 ± 0.06 bB | 6.30 ± 0.06 bA |
| 30 | 6.20 ± 0.01 bB | 6.33 ± 0.01 bA |
| 60 | 6.23 ± 0.04 bB | 6.44 ± 0.06 bA |
| 90 | 6.22 ± 0.02 bB | 6.56 ± 0.05 bA |
| Time (Day) | Electrical Conductivity (µs/cm) * | |
|---|---|---|
| SON | RSON | |
| 01 | 101.47 ± 0.21 aB | 130.20 ± 0.50 aA |
| 15 | 97.33 ± 0.23 bB | 121.53 ± 0.31 bA |
| 30 | 96.07 ± 0.23 bB | 122.60 ± 0.44 bA |
| 60 | 92.10 ± 0.44 bB | 122.23 ± 0.42 bA |
| 90 | 100.53 ± 0.42 aB | 130.00 ± 0.66 aA |
| Treatment | IC50 (µg/mL) * | SI | |
|---|---|---|---|
| HaCaT | A375 | ||
| Attalea funifera seed oil | >500 | >500 | - |
| Resveratrol | 238.86 ± 2.65 | 31.51 ± 1.41 | 7.58 |
| SON | >500 | >500 | - |
| RSON | 1250.00 ± 1.90 | 423.00 ± 1.21 | 2.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dourado, D.; Batista, F.P.R.; Philadelpho, B.O.; de Souza, M.L.; de Cerqueira e Silva, M.B.; de Grandis, R.A.; Miranda, P.A.; Colauto, N.B.; Pereira, D.T.; Formiga, F.R.; et al. Resveratrol-Loaded Attalea funifera Oil Organogel Nanoparticles: A Potential Nanocarrier against A375 Human Melanoma Cells. Int. J. Mol. Sci. 2023, 24, 12112. https://doi.org/10.3390/ijms241512112
Dourado D, Batista FPR, Philadelpho BO, de Souza ML, de Cerqueira e Silva MB, de Grandis RA, Miranda PA, Colauto NB, Pereira DT, Formiga FR, et al. Resveratrol-Loaded Attalea funifera Oil Organogel Nanoparticles: A Potential Nanocarrier against A375 Human Melanoma Cells. International Journal of Molecular Sciences. 2023; 24(15):12112. https://doi.org/10.3390/ijms241512112
Chicago/Turabian StyleDourado, Douglas, Fabiana Pacheco Reis Batista, Biane Oliveira Philadelpho, Myla Lôbo de Souza, Mariana Barros de Cerqueira e Silva, Rone Aparecido de Grandis, Priscila Anjos Miranda, Nelson Barros Colauto, Daniel T. Pereira, Fabio Rocha Formiga, and et al. 2023. "Resveratrol-Loaded Attalea funifera Oil Organogel Nanoparticles: A Potential Nanocarrier against A375 Human Melanoma Cells" International Journal of Molecular Sciences 24, no. 15: 12112. https://doi.org/10.3390/ijms241512112
APA StyleDourado, D., Batista, F. P. R., Philadelpho, B. O., de Souza, M. L., de Cerqueira e Silva, M. B., de Grandis, R. A., Miranda, P. A., Colauto, N. B., Pereira, D. T., Formiga, F. R., Cilli, E. M., Pavan, F. R., Oliveira de Souza, C., & Ferreira, E. d. S. (2023). Resveratrol-Loaded Attalea funifera Oil Organogel Nanoparticles: A Potential Nanocarrier against A375 Human Melanoma Cells. International Journal of Molecular Sciences, 24(15), 12112. https://doi.org/10.3390/ijms241512112