Unravelling the Role of Uncommon Hydrogen Bonds in Cyclodextrin Ferrociphenol Supramolecular Complexes: A Computational Modelling and Experimental Study
Abstract
:1. Introduction
1.1. Cyclodextrins
1.2. Ferrociphenol SuccFerr
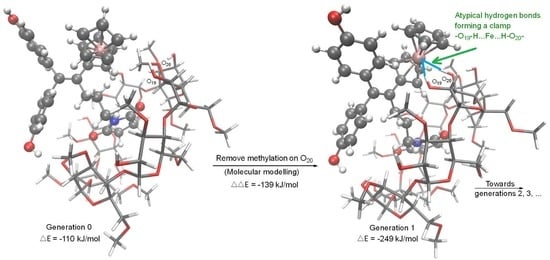
2. Results
2.1. Experiments Prior to Modelling (Proof of Concept)
2.2. Modelling and Web Application
3. Discussion
3.1. Observed Contribution of the Iron Atom to Stability
3.2. Contribution of Methyl Groups to Stability
4. Materials and Methods
4.1. Complexation Studies in Aqueous Phase
4.1.1. Phase Solubility Studies of SuccFerr Complexation
4.1.2. UV–Vis Experiments—Benesi–Hildebrand Method
4.2. Computer Methods
4.2.1. Software
4.2.2. Computational Details
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Wimmer, T. Cyclodextrins. In Ullmann’s Encyclopedia of Industrial Chemistry, 5th ed.; Bohnet, M., Ullmann, F., Eds.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Bakó, P.; Fenichel, L.; Töke, L.; Szente, L.; Szejtli, J. Methylation of cyclodextrins by phase-transfer catalysis. J. Incl. Phenom. Mol. Recogn. Chem. 1994, 18, 307–314. [Google Scholar] [CrossRef]
- Szejtli, J. Cyclodextrins and Their Inclusion Complexes; Akadémiai Kiadó: Budapest, Hungary, 1982. [Google Scholar]
- Arun, R.; Ashok, K.C.K.; Sravanthi, V.V.N.S.S. Cyclodextrins as Drug Carrier Molecule: A Review. Sci. Pharm. 2008, 76, 567–598. [Google Scholar] [CrossRef]
- Conceicao, J.; Adeoye, O.; Cabral-Marques, H.M.; Sousa Lobo, J.M. Hydroxypropyl-β-Cyclodextrin and β-Cyclodextrin as Tablet Fillers for Direct Compression. AAPS PharmSciTech. 2018, 19, 2710–2718. [Google Scholar] [CrossRef] [PubMed]
- Hafner, V.; Czock, D.; Burhenne, J.; Riedel, K.-D.; Bommer, J.; Mikus, G.; Machleidt, C.; Weinreich, T.; Haefeli, W.E. Pharmacokinetics of Sulfobutylether-Beta-Cyclodextrin and Voriconazole in Patients with End-Stage Renal Failure during Treatment with Two Hemodialysis Systems and Hemodiafiltration. Antimicrob. Agents Chemother. 2010, 54, 2596–2602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johannsdottir, S.; Jansook, P.; Stefansson, E.; Loftsson, T. Development of a cyclodextrin-based aqueous cyclosporin A eye drop formulations. Int. J. Pharm. 2015, 493, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Li, Y.; Li, W.; Zhang, H.; Gao, J.; Sun, J.; Yin, X.; Zheng, A. Preparation and evaluation of carfentanil nasal spray employing cyclodextrin inclusion technology. Drug Dev. Ind. Pharm. 2018, 44, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Forgo, P.; Stine, K.J.; D’Souza, V.T. Methods for Selective Modifications of Cyclodextrins. Chem. Rev. 1998, 98, 1977–1996. [Google Scholar] [CrossRef]
- Legrand, F.-X.; Sauthier, M.; Flahaut, C.; Hachani, J.; Elfakir, C.; Fourmentin, S.; Tilloy, S.; Monflier, E. Aqueous hydroformylation reaction mediated by randomly methylated β-cyclodextrin: How substitution degree influences catalytic activity and selectivity. J. Mol. Catal. A Chem. 2009, 303, 72–77. [Google Scholar] [CrossRef]
- Vessières, A.; Top, S.; Pigeon, P.; Hillard, E.A.; Boubekeur, L.; Spera, D.; Jaouen, G. Modification of the Estrogenic Properties of Diphenols by the Incorporation of Ferrocene. Generation of Antiproliferative Effects in Vitro. J. Med. Chem. 2005, 48, 3937–3940. [Google Scholar] [CrossRef] [Green Version]
- Bruyère, C.; Mathieu, V.; Vessières, A.; Pigeon, P.; Top, S.; Jaouen, G.; Kiss, R. Ferrocifen derivatives that induce senescence in cancer cells: Selected examples. J. Inorg. Biochem. 2014, 141, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Tonolo, F.; Salmain, M.; Scalcon, V.; Top, S.; Pigeon, P.; Folda, A.; Caron, B.; McGlinchey, M.J.; Toillon, R.A.; Bindoli, A.; et al. Small Structural Differences Between Two Ferrocenyl Diphenols Determine Large Discrepancies of Reactivity and Biological Effects. ChemMedChem 2019, 14, 1717–1726. [Google Scholar] [CrossRef]
- Wang, Y.; Pigeon, P.; Top, S.; McGlinchey, M.J.; Jaouen, G. Organometallic Antitumor Compounds: Ferrocifens as Precursors to Quinone Methides. Angew. Chem. Int. Ed. Engl. 2015, 54, 10230–10233. [Google Scholar] [CrossRef] [Green Version]
- Richard, M.-A.; Hamels, D.; Pigeon, P.; Top, S.; Dansette, P.M.; Lee, H.Z.S.; Vessières, A.; Mansuy, D.; Jaouen, G. Oxidative Metabolism of Ferrocene Analogues of Tamoxifen: Characterization and Antiproliferative Activities of the Metabolites. ChemMedChem 2015, 10, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Richard, M.-A.; Top, S.; Dansette, P.M.; Pigeon, P.; Vessières, A.; Mansuy, D.; Jaouen, G. Ferrocenyl Quinone Methide-Thiol Adducts as New Antiproliferative Agents: Synthesis, Metabolic Formation from Ferrociphenols, and Oxidative Transformation. Angew. Chem. Int. Ed. Engl. 2016, 128, 10587–10590. [Google Scholar] [CrossRef] [Green Version]
- Citta, A.; Folda, A.; Bindoli, A.; Pigeon, P.; Top, S.; Vessières, A.; Salmain, M.; Jaouen, G.; Rigobello, M.P. Evidence for Targeting Thioredoxin Reductases with Ferrocenyl Quinone Methides. A Possible Molecular Basis for the Antiproliferative Effect of Hydroxyferrocifens on Cancer Cells. J. Med. Chem. 2014, 57, 8849–8859. [Google Scholar] [CrossRef] [Green Version]
- Pigeon, P.; Wang, Y.; Top, S.; Najlaoui, F.; Garcia Alvarez, M.; Bignon, J.; McGlinchey, M.J.; Jaouen, G. A new series of succinimido-ferrociphenols and related heterocyclic species induce strong antiproliferative effects, especially against ovarian cancer cells resistant to cisplatin. J. Med. Chem. 2017, 60, 8358–8368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Pigeon, P.; Top, S.; García, J.; Troufflard, C.; Ciofini, I.; McGlinchey, M.J.; Jaouen, G. Atypical lone pair-π interaction with quinone methides in a series of imido-ferrociphenol anticancer drug candidates. Angew. Chem. Int. Ed. Engl. 2019, 58, 8421–8425. [Google Scholar] [CrossRef]
- Vessières, A.; Quissac, E.; Lemaire, N.; Alentorn, A.; Domeracka, P.; Pigeon, P.; Sanson, M.; Idbaih, A.; Verreault, M. Heterogeneity of Response to Iron-Based Metallodrugs in Glioblastoma Is Associated with Differences in Chemical Structures and Driven by FAS Expression Dynamics and Transcriptomic Subtypes. Int. J. Mol. Sci. 2021, 19, 10404. [Google Scholar] [CrossRef]
- Jaouen, G.; Pigeon, P.; Top, S. Metallocene Derivatives with Anticancer Activity. Patent WO2015063201(A1), 7 May 2015. [Google Scholar]
- Idlas, P.; Lepeltier, E.; Bastiat, G.; Pigeon, P.; McGlinchey, M.J.; Lautram, N.; Vessières, A.; Jaouen, G.; Passirani, C. Physicochemical Characterization of Ferrocifen Lipid Nanocapsules: Customized Drug Delivery Systems Guided by the Molecular Structure. Langmuir 2023, 39, 1885–1896. [Google Scholar] [CrossRef]
- Najlaoui, F.; Pigeon, P.; Aroui, S.; Pezet, M.; Sancey, L.; Marrakchi, N.; Rhouma, A.; Jaouen, G.; De Waard, M.; Busser, B.; et al. Anticancer properties of lipid and poly(ε-caprolactone) nanocapsules loaded with ferrocenyl-tamoxifen derivatives. J. Pharm. Pharmacol. 2018, 70, 1474–1484. [Google Scholar] [CrossRef]
- Idlas, P.; Ladaycia, A.; Némati, F.; Lepeltier, E.; Pigeon, P.; Jaouen, G.; Decaudin, D.; Passirani, C. Ferrocifen Stealth LNCs and Conventional Chemotherapy: A Promising Combination against Multidrug-Resistant Ovarian Adenocarcinoma. Int. J. Pharm. 2022, 626, 122164. [Google Scholar] [CrossRef] [PubMed]
- Najlaoui, F.; Busser, B.; Taïwe, G.S.; Pigeon, P.; Sturm, N.; Giovannini, D.; Marrakchi, N.; Rhouma, A.; Jaouen, G.; Gibaud, S.; et al. Succinimido-Ferrocidiphenol Complexed with Cyclodextrins Inhibits Glioblastoma Tumor Growth In Vitro and In Vivo without Noticeable Adverse Toxicity. Molecules 2022, 14, 4651. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Connors, K. Phase Solubility Techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Najlaoui, F.; Pigeon, P.; Abdelkafi, Z.; Leclerc, S.; Durand, P.; El Ayeb, M.; Marrakchi, N.; Rhouma, A.; Jaouen, G.; Gibaud, S. Phthalimido-ferrocidiphenol cyclodextrin complexes: Characterization and anticancer activity. Int. J. Pharm. 2015, 491, 323–334. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Recherche Data Gouv (to Download File ‘webpages.zip’ and All Webpages within). Available online: https://entrepot.recherche.data.gouv.fr/api/access/datafile/:persistentId?persistentId=doi:10.57745/COGDRQ (accessed on 22 April 2023).
- Pigeon, P. Manipulation of a Cyclodextrin-Ferrociphenol Supramolecular Models Database Using a Web Application; Recherche Data Gouv, V2; Recherche Data Gouv, Ministère de l’enseignement Supérieur et de la Recherche: Paris, France, 2023. [Google Scholar] [CrossRef]
- Pigeon, P. CDModelTree (V1): Manipulation of a Cyclodextrin-Ferrociphenol Supramolecular Models Database Using a PHP Web Application; Software Heritage, INRIA: Le Chesnay-Rocquencourt, France, 2022; Available online: https://hal.science/hal-03991394 (accessed on 22 April 2023).
- XAMPP. Available online: https://www.apachefriends.org (accessed on 22 April 2023).
- Förster, C.; Veit, P.; Ksenofontov, V.; Heinz, K. Diferrocenyl tosyl hydrazone with an ultrastrong NH⋯Fe hydrogen bond as double click switch. Chem. Commun. 2015, 51, 1514–1516. [Google Scholar] [CrossRef] [Green Version]
- Recherche Data Gouv (to Download File ‘Hbonds-1CD.txt’). Available online: https://entrepot.recherche.data.gouv.fr/api/access/datafile/:persistentId?persistentId=doi:10.57745/DQ8QXL (accessed on 22 April 2023).
- Recherche Data Gouv (to Download File ‘Hbonds-2CD.txt’). Available online: https://entrepot.recherche.data.gouv.fr/api/access/datafile/:persistentId?persistentId=doi:10.57745/WK5WE0 (accessed on 22 April 2023).
- Recherche Data Gouv (to Download File ‘DFT.zip’ and All XYZ Models for DFT within). Available online: https://entrepot.recherche.data.gouv.fr/api/access/datafile/:persistentId?persistentId=doi:10.57745/G2KRYZ (accessed on 22 April 2023).
- Buriez, O.; Heldt, J.M.; Labbé, E.; Vessières, A.; Jaouen, G.; Amatore, C. Reactivity and antiproliferative activity of ferrocenyl-tamoxifen adducts with cyclodextrins against hormone-independent breast-cancer cell lines. Chem. Eur. J. 2008, 14, 8195–8203. [Google Scholar] [CrossRef]
- Benesi, H.A.; Hildebrand, J.H. A Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Dotsikas, Y.; Kontopanou, E.; Allagiannis, C.; Loukas, Y.L. Interaction of 6-p-toluidinylnaphthalene-2-sulphonate with β-cyclodextrin. J. Pharm. Biomed. Anal. 2000, 23, 997–1003. [Google Scholar] [CrossRef]
- Yoe, J.H.; Jones, A.L. Colorimetric Determination of Iron with Disodium-1,2-dihydroxybenzene-3,5-disulfonate. Ind. Eng. Chem. Anal. Ed. 1944, 16, 111–115. [Google Scholar] [CrossRef]
- Code::Blocks. Available online: https://www.codeblocks.org (accessed on 22 April 2023).
- Foster, J.P.; Weinhold, F. Natural hybrid orbitals. J. Am. Chem. Soc. 1980, 102, 7211–7218. [Google Scholar] [CrossRef]
- Recherche Data Gouv (to Download File ‘pascal.sql’). Available online: https://entrepot.recherche.data.gouv.fr/api/access/datafile/:persistentId?persistentId=doi:10.57745/38MYXM (accessed on 22 April 2023).
- Recherche Data Gouv (to Download File ‘README.txt’). Available online: https://entrepot.recherche.data.gouv.fr/file.xhtml?persistentId=doi:10.57745/MDGGKE&version=2.0 (accessed on 22 April 2023).






| Series | 1-CD S1 | 1-CD S2 | 1-CD S3 | 1-CD S4 | 1-CD S5 | 1-CD S6 | 1-CD S7 | 1-CD S8 |
|---|---|---|---|---|---|---|---|---|
| Moiety-side A | Fc NS | Fc WS | Ph1 NS | Ph1 WS | Ph2 NS | Ph2 WS | Succ NS | Succ WS |
| βCD G0 B | 2-Me | 3-Me | 2-Me | 3,6-diMe | 2-Me | 6-Me | 2,3-diMe | 3,6-diMe |
| ∆E G0 C | −122 | −83 | −111 | −141 | −48 | −49 | −161 | −111 |
| Average ∆E D | −154 | −144 | −127 | −177 | −68 | −98 | −187 | −274 |
| Best ∆E E | −194 | −261 | −142 | −204 | −127 | −169 | −219 | −302 |
| Max G/Gbest F | 33/29 | 19/18 | 13/12 | 13/10 | 12/11 | 4/4 | 18/17 | 21/16 |
| Average Me G | 1.4 | 1.07 | 1.09 | 1.73 | 1.04 | 1.31 | 1.34 | 0.98 |
| Me Domain H | 0.6–2.4 | 0.3–2.0 | 0.6–1.6 | 1.1–2.3 | 0.6–1.3 | 1.0–1.6 | 0.6–2.3 | 0.3–2.1 |
| Fe-H(CD) I | 191(0) 1(0) | 497(118) 145(0) | 0(0) 0(0) | 60(0) 0(0) | 0(0) 0(0) | 1(0) 0(0) | 929(0) 0(0) | 3414(1664) 2(0) |
| NbMeFc J | 1.44 0.89 | 1.60 2.37 | 0.53 1.57 | 1.26 2.88 | 0.02 0.14 | 1.05 0.52 | 1.41 1.30 | 0.93 1.33 |
| Samples K | 2726 | 1994 | 571 | 326 | 187 | 79 | 1106 | 1903 |
| ID G0 L | 1 | 2727 | 4721 | 5292 | 5618 | 5805 | 5884 | 6990 |
| Free CDs M | 2690 | 1877 | 550 | 305 | 166 | 57 | 1084 | 1872 |
| Series | 2-CD S1 | 2-CD S2 | 2-CD S3 | 2-CD S4 | ||||
| Moiety-side 1 A | Fc NS | Fc NS | Fc NS | Succ NS | ||||
| Moiety-side 2 A | Succ WS | Ph2 WS | Ph1 WS | Ph1 WS | ||||
| βCD G0 B | 3-Me | 3-Me | 2,3-diMe | 2-Me | ||||
| ∆E G0 C | −278 | −208 | −203 | −236 | ||||
| Average ∆E D | −271 | −293 | −248 | −283 | ||||
| Best ∆E E | −330 | −338 | −296 | −326 | ||||
| Max G/Gbest F | 22/19 | 15/15 | 12/11 | 27/26 | ||||
| Average Me G | 1.33, 1.36 | 1.03, 1.16 | 1.94, 1.88 | 0.97, 0.81 | ||||
| Me Domain H | 0.9–1.9 | 0.6–1.4 | 1.6–2.3 | 0.4–1.4 | ||||
| Fe-H(CD) I | 790(0) 58(0) | 534(0) 24(0) | 367(0) 48(0) | 1175(0) 3(0) | ||||
| NbMeFc J | 1.11 2.05 | 0.97 1.58 | 0.94 0.87 | 1.14 1.80 | ||||
| Samples K | 1138 | 642 | 449 | 1229 | ||||
| ID G0 L | 1 | 1139 | 1781 | 2230 | ||||
| Free CDs M | 495 | 253 | 195 | 451 | ||||
| NBO Donor | NBO Acceptor | E(2) (kJ/mol) |
|---|---|---|
| Fe ----- H—O19 | ||
| LPAll (Fe) | RydbergAll (H) | 1.13 |
| LPAll (Fe) | σ*All (H—O) | 3.80 |
| LP*All (Fe) | RydbergAll (H) | 56.77 |
| LP*All (Fe) | RydbergAll (O) | 9.23 |
| LP*All (Fe) | σ*All (H—O) | 4.48 |
| σAll (H—O) | LP*All (Fe) | 9.83 |
| σAll (H—O) | RydbergAll (Fe) | 0.29 |
| LPAll (O) | LP*All (Fe) | 0.92 |
| Fe ----- H—O20 | ||
| CRAll (Fe) | RydbergAll (H) | 0.21 |
| LPAll (Fe) | RydbergAll (H) | 3.14 |
| LPAll (Fe) | σ*All (H—O) | 2.90 |
| LP*All (Fe) | RydbergAll (H) | 98.40 |
| LP*All (Fe) | σ*All (H—O) | 4.43 |
| σAll (H—O) | LP*All (Fe) | 8.61 |
| σAll (H—O) | RydbergAll (Fe) | 0.50 |
| LPAll (O) | LP*All (Fe) | 1.05 |
| Type of Hydrogen Bond | Length PM3 (Å) | Length DFT (Å) | Angle PM3 (°) | Angle DFT (°) |
|---|---|---|---|---|
| O19-H…Fe | 1.933 | 2.774 | 167 | 159 |
| O20-H…Fe | 1.896 | 2.694 | 177 | 166 |
| C=O(1)…H(CD) | 1.910 | 2.010 | 148 | 128 |
| C=O(2)…H(CD) (first) | 1.838 | 1.864 | 166 | 164 |
| C=O(2)…H(CD) (second) | 1.858 | 2.087 | 162 | 163 |
| H(Ph2)…O(CD) | 1.843 | 1.751 | 167 | 173 |
| H…O intra CD (1) | 1.863 | 1.874 | 163 | 173 |
| H…O intra CD (2) | 1.825 | 1.815 | 168 | 175 |
| H…O intra CD (3) | 1.862 | 1.868 | 172 | 164 |
| H…O intra CD (4) | 1.816 | 1.836 | 174 | 175 |
| H…O intra CD (5) | 2.474 | 2.294 | 108 | 111 |
| H…O intra CD (6) | 1.862 | 1.868 | 172 | 164 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pigeon, P.; Najlaoui, F.; McGlinchey, M.J.; Sanz García, J.; Jaouen, G.; Gibaud, S. Unravelling the Role of Uncommon Hydrogen Bonds in Cyclodextrin Ferrociphenol Supramolecular Complexes: A Computational Modelling and Experimental Study. Int. J. Mol. Sci. 2023, 24, 12288. https://doi.org/10.3390/ijms241512288
Pigeon P, Najlaoui F, McGlinchey MJ, Sanz García J, Jaouen G, Gibaud S. Unravelling the Role of Uncommon Hydrogen Bonds in Cyclodextrin Ferrociphenol Supramolecular Complexes: A Computational Modelling and Experimental Study. International Journal of Molecular Sciences. 2023; 24(15):12288. https://doi.org/10.3390/ijms241512288
Chicago/Turabian StylePigeon, Pascal, Feten Najlaoui, Michael James McGlinchey, Juan Sanz García, Gérard Jaouen, and Stéphane Gibaud. 2023. "Unravelling the Role of Uncommon Hydrogen Bonds in Cyclodextrin Ferrociphenol Supramolecular Complexes: A Computational Modelling and Experimental Study" International Journal of Molecular Sciences 24, no. 15: 12288. https://doi.org/10.3390/ijms241512288
APA StylePigeon, P., Najlaoui, F., McGlinchey, M. J., Sanz García, J., Jaouen, G., & Gibaud, S. (2023). Unravelling the Role of Uncommon Hydrogen Bonds in Cyclodextrin Ferrociphenol Supramolecular Complexes: A Computational Modelling and Experimental Study. International Journal of Molecular Sciences, 24(15), 12288. https://doi.org/10.3390/ijms241512288