Enhancing Nasopharyngeal Carcinoma Cell Separation with Selective Fibronectin Coating and Topographical Modification on Polydimethylsiloxane Scaffold Platforms
Abstract
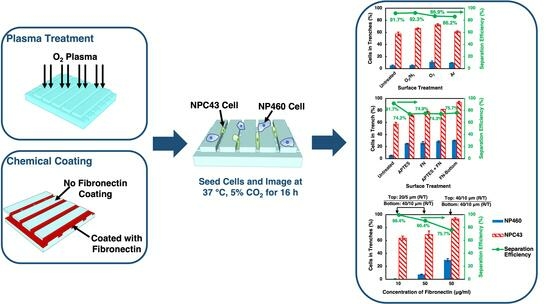
:1. Introduction
2. Results and Discussion
2.1. NP460 and NPC43 Cells on Plasma-Treated Surfaces
2.1.1. Surface Energy and Roughness of Plasma-Treated Surfaces
2.1.2. Effects of Plasma Treatments on Cell Motility
2.1.3. Effects of Plasma Treatments on Cell Morphology and Traversing Behaviors
2.2. NP460 and NPC43 Cells on Chemically Coated Surfaces
2.2.1. Characterization of Coating on Platforms
2.2.2. Effects of Chemical Coatings on Cell Motility
2.2.3. Effects of Chemical Coatings on Cell Morphology and Traversing Behavior
2.3. Separation of NP460 Cells from NPC43 Cells by Platform Design and Chemical Coating
3. Materials and Methods
3.1. Fabrication Technology for Biomimetic Two-Layer Scaffold Platforms
3.2. Surface Modification of PDMS Platforms
3.3. Nasopharyngeal Epithelial and Carcinoma Cell Culture
3.4. Fluorescent Imaging of Fibronectin
3.5. Time-Lapse Imaging and Data Analysis
3.6. Scanning Electron Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, W.I.; Sham, J.S. Nasopharyngeal Carcinoma. Lancet 2005, 365, 2041–2054. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.T.; SchÄfer, R.; Paul, A.; Gerber, A.; Ziemer, G.; Wendel, H.P. A New Technique for the Isolation and Surface Immobilization of Mesenchymal Stem Cells from Whole Bone Marrow Using High-Specific DNA Aptamers. Stem Cells 2006, 24, 2220–2231. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, M.J.; Tomlinson, S.; Yang, X.B.; Kirkham, J. Cell Separation: Terminology and Practical Considerations. J. Tissue Eng. 2013, 4, 2041731412472690. [Google Scholar] [CrossRef] [PubMed]
- Yousuff, C.M.; Ho, E.T.W.; Hussain, K.I.; Hamid, N.H.B. Microfluidic Platform for Cell Isolation and Manipulation Based on Cell Properties. Micromachines 2017, 8, 15. [Google Scholar] [CrossRef]
- Lam, B.P.; Lam, Y.W.; Pang, S.W. Using Biomimetic Scaffold Platform to Detect Growth Factor Induced Changes in Migration Dynamics of Nasopharyngeal Epithelial Cells. IEEE Access 2020, 8, 187553–187563. [Google Scholar] [CrossRef]
- Trantidou, T.; Elani, Y.; Parsons, E.; Ces, O. Hydrophilic Surface Modification of PDMS for Droplet Microfluidics Using a Simple, Quick, and Robust Method Via Pva Deposition. Microsyst. Nanoeng. 2017, 3, 16091. [Google Scholar] [CrossRef]
- Kuddannaya, S.; Chuah, Y.J.; Lee, M.H.A.; Menon, N.V.; Kang, Y.; Zhang, Y. Surface Chemical Modification of Poly(dimethylsiloxane) for the Enhanced Adhesion and Proliferation of Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2013, 5, 9777–9784. [Google Scholar] [CrossRef]
- Chuah, Y.J.; Kuddannaya, S.; Lee, M.H.A.; Zhang, Y.; Kang, Y. The Effects of Poly(dimethylsiloxane) Surface Silanization on the Mesenchymal Stem Cell Fate. Biomater. Sci. 2015, 3, 383–390. [Google Scholar] [CrossRef]
- Liu, W.; Zhan, J.; Su, Y.; Wu, T.; Wu, C.; Ramakrishna, S.; Mo, X.; Al-Deyab, S.S.; El-Newehy, M. Effects of Plasma Treatment to Nanofibers on Initial Cell Adhesion and Cell Morphology. Colloids Surf. B 2014, 113, 101–106. [Google Scholar] [CrossRef]
- Tee, J.Y.; Mackay-Sim, A. Directional Persistence of Cell Migration in Schizophrenia Patient-Derived Olfactory Cells. Int. J. Mol. Sci. 2021, 22, 9177. [Google Scholar] [CrossRef]
- Paguirigan, A.L.; Beebe, D.J. Microfluidics Meet Cell Biology: Bridging the Gap by Validation and Application of Microscale Techniques for Cell Biological Assays. Bioessays 2008, 30, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Sudo, R.; Mack, P.J.; Wan, C.-R.; Vickerman, V.; Kamm, R.D. Cell Migration into Scaffolds under Co-Culture Conditions in a Microfluidic Platform. Lab A Chip 2009, 9, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-Y.; Chien, Y.; Chuang, J.-H.; Chang, C.-C.; Yang, Y.-P.; Lai, Y.-H.; Lo, W.-L.; Chien, K.-H.; Huo, T.-I.; Wang, C.-Y. Development of a Graphene Oxide-Incorporated Polydimethylsiloxane Membrane with Hexagonal Micropillars. Int. J. Mol. Sci. 2018, 19, 2517. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Z.; Pang, S.W. Effects of Three-Dimensional Platform Stiffness and Layer Dimensions on Separation of Carcinoma Cells. Engineering 2021, 7, 1424–1433. [Google Scholar] [CrossRef]
- Saez, A.; Ghibaudo, M.; Buguin, A.; Silberzan, P.; Ladoux, B. Rigidity-Driven Growth and Migration of Epithelial Cells on Microstructured Anisotropic Substrates. Proc. Natl. Acad. Sci. USA 2007, 104, 8281–8286. [Google Scholar] [CrossRef]
- Torino, S.; Corrado, B.; Iodice, M.; Coppola, G. PDMS-Based Microfluidic Devices for Cell Culture. Inventions 2018, 3, 65. [Google Scholar] [CrossRef]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2021, 13, 2. [Google Scholar] [CrossRef]
- Akther, F.; Yakob, S.B.; Nguyen, N.-T.; Ta, H.T. Surface Modification Techniques for Endothelial Cell Seeding in PDMS Microfluidic Devices. Biosensors 2020, 10, 182. [Google Scholar] [CrossRef]
- Ren, X.; Bachman, M.; Sims, C.; Li, G.; Allbritton, N. Electroosmotic Properties of Microfluidic Channels Composed of Poly(dimethylsiloxane). J. Chromatogr. B 2001, 762, 117–125. [Google Scholar] [CrossRef]
- Griffin, M.; Palgrave, R.; Baldovino-Medrano, V.G.; Butler, P.E.; Kalaskar, D.M. Argon Plasma Improves the Tissue Integration and Angiogenesis of Subcutaneous Implants by Modifying Surface Chemistry and Topography. Int. J. Nanomed. 2018, 13, 6123. [Google Scholar] [CrossRef]
- Wang, F.; Ling, B.; Li, Q.; Abouhany, R. Dual Roles of 3-Aminopropyltriethoxysilane in Preparing Molecularly Imprinted Silica Particles for Specific Recognition of Target Molecules. RSC Adv. 2020, 10, 20368–20373. [Google Scholar] [CrossRef] [PubMed]
- Chuah, Y.J.; Koh, Y.T.; Lim, K.; Menon, N.V.; Wu, Y.; Kang, Y. Simple Surface Engineering of Polydimethylsiloxane with Polydopamine for Stabilized Mesenchymal Stem Cell Adhesion and Multipotency. Sci. Rep. 2015, 5, 18162. [Google Scholar] [CrossRef]
- Formentín, P.; Catalán, Ú.; Pol, L.; Fernández-Castillejo, S.; Solà, R.; Marsal, L. Collagen and Fibronectin Surface Modification of Nanoporous Anodic Alumina and Macroporous Silicon for Endothelial Cell Cultures. J. Biol. Eng. 2018, 12, 21. [Google Scholar] [CrossRef]
- Hsiao, C.-T.; Cheng, H.-W.; Huang, C.-M.; Li, H.-R.; Ou, M.-H.; Huang, J.-R.; Khoo, K.-H.; Yu, H.W.; Chen, Y.-Q.; Wang, Y.-K.; et al. Fibronectin in Cell Adhesion and Migration Via N-Glycosylation. Oncotarget 2017, 8, 70653–70668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Z.; Pang, S.W. Separation of Nasopharyngeal Epithelial Cells from Carcinoma Cells on 3D Scaffold Platforms. Biotechnol. Bioeng. 2021, 118, 1444–1455. [Google Scholar] [CrossRef]
- Wang, M.T.; Pang, S.W. Controlled Scaffold Platform Designs on Nasopharyngeal Carcinoma Cell Separation. IEEE Access 2021, 9, 113813–113822. [Google Scholar] [CrossRef]
- Refaaq, F.; Chen, X.; Pang, S.W. Effects of Topographical Guidance Cues on Osteoblast Cell Migration. Sci. Rep. 2020, 10, 20003. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, W.; Pang, S.W. Traversing Behavior of Tumor Cells in Three-Dimensional Platforms with Different Topography. PLoS ONE 2020, 15, e0234482. [Google Scholar]
- Tang, Q.; Qian, W.; Xu, Y.; Gopalakrishnan, S.; Wang, J.; Lam, Y.; Pang, S.W. Control of Cell Migration Direction by Inducing Cell Shape Asymmetry with Patterned Topography. J. Biomed. Mater. Res. Part A 2015, 103, 2383–2393. [Google Scholar] [CrossRef]
- Faucheux, N.; Schweiss, R.; Lützow, K.; Werner, C.; Groth, T. Self-Assembled Monolayers with Different Terminating Groups as Model Substrates for Cell Adhesion Studies. Biomaterials 2004, 25, 2721–2730. [Google Scholar] [CrossRef]
- Keselowsky, B.G.; Collard, D.M.; García, A.J. Surface Chemistry Modulates Focal Adhesion Composition and Signaling through Changes in Integrin Binding. Biomaterials 2004, 25, 5947–5954. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, E.; Duval, J.L.; Pezron, I.; Nadaud, F. Behaviors of Liver and Kidney Explants from Chicken Embryos inside Plasma Treated PDMS Microchannels. Mater. Sci. Eng. C 2009, 29, 861–868. [Google Scholar] [CrossRef]
- Li, D.; Neumann, A. Equation of State for Interfacial Tensions of Solid-Liquid Systems. Adv. Colloid Interface Sci. 1992, 39, 299–345. [Google Scholar] [CrossRef]
- Keshel, S.H.; Azhdadi, S.N.K.; Asefnezhad, A.; Sadraeian, M.; Montazeri, M.; Biazar, E. The Relationship between Cellular Adhesion and Surface Roughness for Polyurethane Modified by Microwave Plasma Radiation. Int. J. Nanomed. 2011, 6, 641. [Google Scholar]
- Abou Rich, S.; Dufour, T.; Leroy, P.; Nittler, L.; Pireaux, J.J.; Reniers, F. Low-Density Polyethylene Films Treated by an Atmospheric Ar–O2 Post-Discharge: Functionalization, Etching, Degradation and Partial Recovery of the Native Wettability State. J. Phys. D Appl. Phys. 2014, 47, 065203. [Google Scholar] [CrossRef]
- Puech, M.; Thevenoud, J.-M.; Gruffat, J.; Launay, N.; Arnal, N.; Godinat, P. Fabrication of 3D Packaging TSV Using DRIE. In Proceedings of the 2008 Symposium on Design, Test, Integration and Packaging of MEMS/MOEMS, Nice, France, 9–11 April 2008; pp. 109–114. [Google Scholar]
- Nascimento, F.D.; Quade, A.; Canesqui, M.A.; Kostov, K.G. Different Configurations of Transferred Atmospheric Pressure Plasma Jet and Their Application to Polymer Treatment. Contrib. Plasma Phys. 2023, 63, e202200055. [Google Scholar] [CrossRef]
- Yang, Y.; Kulangara, K.; Lam, R.T.; Dharmawan, R.; Leong, K.W. Effects of Topographical and Mechanical Property Alterations Induced by Oxygen Plasma Modification on Stem Cell Behavior. ACS Nano 2012, 6, 8591–8598. [Google Scholar] [CrossRef]
- Pranda, M.A.; Murugesan, B.J.; Knoll, A.J.; Oehrlein, G.S.; Stroka, K.M. Sensitivity of Tumor Versus Normal Cell Migration and Morphology to Cold Atmospheric Plasma-Treated Media in Varying Culture Conditions. Plasma Process. Polym. 2020, 17, 1900103. [Google Scholar] [CrossRef]
- Zuchowska, A.; Kwiatkowski, P.; Jastrzebska, E.; Chudy, M.; Dybko, A.; Brzozka, Z. Adhesion of Mrc-5 and A549 Cells on Poly(dimethylsiloxane) Surface Modified by Proteins. Electrophoresis 2016, 37, 536–544. [Google Scholar] [CrossRef]
- Pathak, A.; Kumar, S. Independent Regulation of Tumor Cell Migration by Matrix Stiffness and Confinement. Proc. Natl. Acad. Sci. USA 2012, 109, 10334–10339. [Google Scholar] [CrossRef]
- van Horssen, R.; Galjart, N.; Rens, J.A.; Eggermont, A.M.; Hagen, T.L.T. Differential Effects of Matrix and Growth Factors on Endothelial and Fibroblast Motility: Application of a Modified Cell Migration Assay. J. Cell. Biochem. 2006, 99, 1536–1552. [Google Scholar] [CrossRef] [PubMed]
- Liberio, M.S.; Sadowski, M.C.; Soekmadji, C.; Davis, R.A.; Nelson, C.C. Differential Effects of Tissue Culture Coating Substrates on Prostate Cancer Cell Adherence, Morphology and Behavior. PLoS ONE 2014, 9, e112122. [Google Scholar] [CrossRef] [PubMed]
- Makamba, H.; Kim, J.H.; Lim, K.; Park, N.; Hahn, J.H. Surface Modification of Poly(dimethylsiloxane) Microchannels. Electrophoresis 2003, 24, 3607–3619. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.H.; Lee, J.; Sen, P.N. Long-Term Retention of Hydrophilic Behavior of Plasma Treated Polydimethylsiloxane (PDMS) Surfaces Stored under Water and Luria-Bertani Broth. Sens. Actuators A 2012, 181, 33–42. [Google Scholar] [CrossRef]
- Tsang, C.; Liu, Z.; Zhang, W.; You, C.; Jones, G.; Tsao, S.; Pang, S.W. Integration of Biochemical and Topographic Cues for the Formation and Spatial Distribution of Invadosomes in Nasopharyngeal Epithelial Cells. Acta Biomater. 2020, 101, 168–182. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.T.; Pang, S.W. Enhancing Nasopharyngeal Carcinoma Cell Separation with Selective Fibronectin Coating and Topographical Modification on Polydimethylsiloxane Scaffold Platforms. Int. J. Mol. Sci. 2023, 24, 12409. https://doi.org/10.3390/ijms241512409
Wang MT, Pang SW. Enhancing Nasopharyngeal Carcinoma Cell Separation with Selective Fibronectin Coating and Topographical Modification on Polydimethylsiloxane Scaffold Platforms. International Journal of Molecular Sciences. 2023; 24(15):12409. https://doi.org/10.3390/ijms241512409
Chicago/Turabian StyleWang, M. T., and S. W. Pang. 2023. "Enhancing Nasopharyngeal Carcinoma Cell Separation with Selective Fibronectin Coating and Topographical Modification on Polydimethylsiloxane Scaffold Platforms" International Journal of Molecular Sciences 24, no. 15: 12409. https://doi.org/10.3390/ijms241512409
APA StyleWang, M. T., & Pang, S. W. (2023). Enhancing Nasopharyngeal Carcinoma Cell Separation with Selective Fibronectin Coating and Topographical Modification on Polydimethylsiloxane Scaffold Platforms. International Journal of Molecular Sciences, 24(15), 12409. https://doi.org/10.3390/ijms241512409