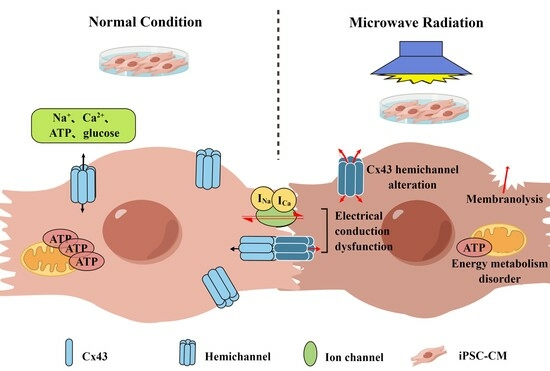
Role of Cx43 in iPSC-CM Damage Induced by Microwave Radiation
Abstract
:1. Introduction
2. Results
2.1. Identification of Cell Differentiation
2.2. Cell Membrane Damage after Microwave Radiation
2.2.1. Cell Activity Decreased and Membrane Permeability Increased
2.2.2. The Contents of Myocardial Enzymes and Injury Markers in iPSC-CMs Increased
2.2.3. Membrane Ultrastructure Damage in iPSC-CMs
2.3. Energy Metabolism Disorder of iPSC-CMs after Microwave Radiation
2.3.1. Abnormal Mitochondrial Ultrastructure
2.3.2. Reduced Mitochondrial Respiration Capacity
2.3.3. Abnormal Glycolytic Capacity
2.4. Conduction Damage in iPSC-CMs after Microwave Radiation
2.4.1. Ultrastructure of ID
2.4.2. Electrical Conduction Dysfunction
2.4.3. Changes in Cx43 Expression in iPSC-CMs after Microwave Exposure
2.4.4. Changes in Cx43 Distribution in iPSC-CMs after Microwave Exposure
3. Discussion
4. Materials and Methods
4.1. Cell Source and Culture Method
4.2. Cell Identification
4.3. Groups and Exposure Methods
4.4. FCM
4.5. ELISA
4.6. SEM
4.7. TEM
4.8. Measurement of OCR and ECAR
4.9. Electrical Mapping Recording
4.10. Wb
4.11. qRT-PCR
4.12. Immunofluorescence
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ao, W.; Fu, J.; Mao, X.; Kang, Q.; Ran, C.; Liu, Y.; Zhang, H.; Gao, Z.; Li, J.; Liu, G.; et al. Microwave assisted preparation of activated carbon from biomass: A review. Renew. Sustain. Energy Rev. 2018, 92, 958–979. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, L.; Liu, F.; Liu, H.; Zhang, N.; Zhu, Y. Energy, environment and economy assessment of medical waste disposal technologies in China. Sci. Total Environ. 2021, 796, 148964. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C. Microwave thermoacoustic tomographic (MTT) imaging. Phys. Med. Biol. 2021, 66, 10tr02. [Google Scholar] [CrossRef] [PubMed]
- Idrus, I.N.; Faruque, M.R.I.; Abdullah, S.; Khandaker, M.U.; Tamam, N.; Sulieman, A. An Oval-Square Shaped Split Ring Resonator Based Left-Handed Metamaterial for Satellite Communications and Radar Applications. Micromachines 2022, 13, 578. [Google Scholar] [CrossRef] [PubMed]
- Zaroushani, V.; Khajehnasiri, F. Long Term Exposure to Microwave Radiation in Children Due to COVID-19 Pandemic; a Carcinogen Challenge. J. Res. Health Sci. 2020, 20, e00501. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Liu, W.; Liu, Y.; Liu, Y.; Xu, Z.; Ye, Y.; Zhou, H.; Deng, H.; Zuo, H.; Yang, H.; et al. Effects of Nonthermal Radiofrequency Stimulation on Neuronal Activity and Neural Circuit in Mice. Adv. Sci. 2023, 10, e2205988. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Hu, S.H.; Tan, S.Z.; Zhang, B.; Zhou, H.M.; Peng, R.Y. Real-time Microwave Exposure Induces Calcium Efflux in Primary Hippocampal Neurons and Primary Cardiomyocytes. Biomed. Environ. Sci. 2018, 31, 561–571. [Google Scholar] [CrossRef]
- Bekhite, M.; Gonzalez-Delgado, A.; Huebner, S.; Haxhikadrija, P.; Kretzschmar, T.; Mueller, T.; Wu, J.M.F.; Bekfani, T.; Franz, M.; Wartenberg, M.; et al. The role of ceramide accumulation in human induced pluripotent stem cell-derived cardiomyocytes on mitochondrial oxidative stress and mitophagy. Free Radic. Biol. Med. 2021, 167, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xue, Y.; Pan, T.; Zhu, X.; Chong, H.; Xu, C.; Fan, F.; Cao, H.; Zhang, B.; Pan, J.; et al. Epicardial injection of allogeneic human-induced-pluripotent stem cell-derived cardiomyocytes in patients with advanced heart failure: Protocol for a phase I/IIa dose-escalation clinical trial. BMJ Open 2022, 12, e056264. [Google Scholar] [CrossRef]
- Nielsen, M.S.; van Opbergen, C.J.M.; van Veen, T.A.B.; Delmar, M. The intercalated disc: A unique organelle for electromechanical synchrony in cardiomyocytes. Physiol. Rev. 2023, 103, 2271–2319. [Google Scholar] [CrossRef]
- Yin, Y.; Xu, X.P.; Gao, Y.B.; Wang, J.; Yao, B.W.; Zhao, L.; Wang, H.Y.; Wang, H.; Dong, J.; Zhang, J.; et al. Abnormal Expression of Connexin43 in Cardiac Injury Induced by S-Band and X-Band Microwave Exposure in Rats. J. Immunol. Res. 2021, 2021, 3985697. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, X.; Yin, Y.; Yao, B.; Dong, J.; Zhao, L.; Wang, H.; Wang, H.; Zhang, J.; Peng, R. Physiological and Psychological Stress of Microwave Radiation-Induced Cardiac Injury in Rats. Int. J. Mol. Sci. 2023, 24, 6237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.M.; Navara, R.; Yin, T.; Szymanski, J.; Goldsztejn, U.; Kenkel, C.; Lang, A.; Mpoy, C.; Lipovsky, C.E.; Qiao, Y.; et al. Cardiac radiotherapy induces electrical conduction reprogramming in the absence of transmural fibrosis. Nat. Commun. 2021, 12, 5558. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sutani, A.; Kaneko, R.; Takeuchi, J.; Sasano, T.; Kohda, T.; Ihara, K.; Takahashi, K.; Yamazoe, M.; Morio, T.; et al. In vitro generation of functional murine heart organoids via FGF4 and extracellular matrix. Nat. Commun. 2020, 11, 4283. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.F.; Hather, C.; Conteh, J.S.; Zhang, J.; Popa, R.G.; Owen, A.W.; Jonas, C.L.; Choi, H.; Daniel, R.M.; Lloyd, D.; et al. Non-thermal disruption of β-adrenergic receptor-activated Ca2+ signalling and apoptosis in human ES-derived cardiomyocytes by microwave electric fields at 2.4 GHz. Biochem. Biophys. Res. Commun. 2023, 661, 89–98. [Google Scholar] [CrossRef]
- Varghese, R.; Majumdar, A.; Kumar, G.; Shukla, A. Rats exposed to 2.45GHz of non-ionizing radiation exhibit behavioral changes with increased brain expression of apoptotic caspase 3. Pathophysiology 2018, 25, 19–30. [Google Scholar] [CrossRef]
- Duan, K.Z.; Gu, Q.H.; Petralia, R.S.; Wang, Y.X.; Panja, D.; Liu, X.; Lehmann, M.L.; Zhu, H.W.; Zhu, J.; Li, Z. Mitophagy in the basolateral amygdala mediates increased anxiety induced by aversive social experience. Neuron 2021, 109, 3793–3809. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q. Using Seahorse Machine to Measure OCR and ECAR in Cancer Cells. Cancer Metab. Methods Protoc. 2019, 1928, 353–363. [Google Scholar] [CrossRef]
- Neogi, U.; Elaldi, N.; Appelberg, S.; Ambikan, A.; Kennedy, E.; Dowall, S.; Bagci, B.K.; Gupta, S.; Rodriguez, J.E.; Svensson-Akusjärvi, S.; et al. Multi-omics insights into host-viral response and pathogenesis in Crimean-Congo hemorrhagic fever viruses for novel therapeutic target. Elife 2022, 11, e76071. [Google Scholar] [CrossRef]
- Wang, T.; Xiao, Y.; Zhang, J.; Jing, F.; Zeng, G. Dynamic regulation of HIF-1 signaling in the rhesus monkey heart after ischemic injury. BMC Cardiovasc. Disord. 2022, 22, 407. [Google Scholar] [CrossRef]
- Moise, N.; Struckman, H.L.; Dagher, C.; Veeraraghavan, R.; Weinberg, S.H. Intercalated disk nanoscale structure regulates cardiac conduction. J. Gen. Physiol. 2021, 153, e202112897. [Google Scholar] [CrossRef] [PubMed]
- Lousinha, A.; Pereira, G.; Borrecho, G.; Brito, J.; de Carvalho, A.O.; Freitas, D.; Oliveira, P.; Oliveira, M.J.R.; Antunes, E. Atrial fibrosis and decreased connexin 43 in rat hearts after exposure to high-intensity infrasound. Exp. Mol. Pathol. 2020, 114, 104409. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Ren, D.Q.; Jin, J.; Huang, X.F.; Liu, R.H. Effect the change of EMP on the spatial distribution of the link-structure of rat’s myocardial intercalated disks. China Practical Med. 2008, 18, 1–3. [Google Scholar] [CrossRef]
- Hu, C.; Zuo, H.; Li, Y. Effects of Radiofrequency Electromagnetic Radiation on Neurotransmitters in the Brain. Front. Public Health 2021, 9, 691880. [Google Scholar] [CrossRef] [PubMed]
- Magiera, A.; Solecka, J. Mobile telephony and its effects on human health. Rocz. Panstw. Zakl. Hig. 2019, 70, 225–234. [Google Scholar] [CrossRef]
- Falcioni, L.; Bua, L.; Tibaldi, E.; Lauriola, M.; De Angelis, L.; Gnudi, F.; Mandrioli, D.; Manservigi, M.; Manservisi, F.; Manzoli, I.; et al. Report of final results regarding brain and heart tumors in Sprague-Dawley rats exposed from prenatal life until natural death to mobile phone radiofrequency field representative of a 1.8 GHz GSM base station environmental emission. Environ. Res 2018, 165, 496–503. [Google Scholar] [CrossRef]
- Schneider, R. Mobile phone induced EMF stress is reversed upon the use of protective devices: Results from two experiments testing different boundary conditions. Electromagn. Biol. Med. 2022, 41, 429–438. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.; Dong, J.; Wang, S.; Yao, B.; Zhang, J.; Hu, S.; Xu, X.; Zuo, H.; Wang, L.; et al. The compound Chinese medicine “Kang Fu Ling” protects against high power microwave-induced myocardial injury. PLoS ONE 2014, 9, e101532. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, R.Y.; Gao, Y.B.; Wang, S.M.; Yang, L.L.; Zhao, L.; Dong, J.; Yao, B.W.; Chang, G.M.; Xiong, L. AduoLa Fuzhenglin down-regulates microwave-induced expression of β1-adrenergic receptor and muscarinic type 2 acetylcholine receptor in myocardial cells of rats. Biomed. Environ. Sci. 2014, 27, 204–207. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Yao Bin, W.; Xu Xin, P.; Wang, H.; Zhao, L.; Dong, J.; Wang Hao, Y.; Tan Sheng, Z.; Peng Rui, Y. Dose-Dependent, Frequency-Dependent, and Cumulative Effects on Cardiomyocyte Injury and Autophagy of 2.856 GHz and 1.5 GHz Microwave in Wistar Rats. Biomed. Environ. Sci. 2022, 35, 351–355. [Google Scholar] [CrossRef]
- Chen, Z.F.; Xian, W.Y.; Bellin, M.; Dorn, T.; Tian, Q.H.; Goedel, A.; Dreizehnter, L.; Schneider, C.M.; Oostwaard, D.V.; Ng, J.K.M.; et al. Subtype-specific promoter-driven action potential imaging for precise disease modelling and drug testing in hiPSC-derived cardiomyocytes. Eur. Heart J. 2017, 38, 292–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, X.; Xu, W.; Zhang, H.; Wang, Q.; Yu, J.; Zhang, R.; Chen, Y.; Xia, Y.; Wang, J.; Wang, D. Transplantation of human induced pluripotent stem cell-derived cardiomyocytes improves myocardial function and reverses ventricular remodeling in infarcted rat hearts. Stem Cell Res. Ther. 2020, 11, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, M.; Miyagawa, S.; Saito, A.; Fukushima, S.; Harada, A.; Ito, E.; Ohashi, F.; Watabe, T.; Hatazawa, J.; Matsuura, K.; et al. Transplantation of Human-induced Pluripotent Stem Cell-derived Cardiomyocytes Is Superior to Somatic Stem Cell Therapy for Restoring Cardiac Function and Oxygen Consumption in a Porcine Model of Myocardial Infarction. Transplantation 2019, 103, 291–298. [Google Scholar] [CrossRef]
- Narita, H.; Shima, F.; Yokoyama, J.; Miyagawa, S.; Tsukamoto, Y.; Takamura, Y.; Hiura, A.; Fukumoto, K.; Chiba, T.; Watanabe, S.; et al. Engraftment and morphological development of vascularized human iPS cell-derived 3D-cardiomyocyte tissue after xenotransplantation. Sci. Rep. 2017, 7, 13708. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Xu, X.; Gao, Y.; Wang, J.; Yin, Y.; Yao, B.; Zhao, L.; Wang, H.; Wang, H.; Dong, J.; et al. Hsp72-Based Effect and Mechanism of Microwave Radiation-Induced Cardiac Injury in Rats. Oxidative Med. Cell. Longev. 2022, 2022, 7145415. [Google Scholar] [CrossRef] [PubMed]
- Pernas, L.; Scorrano, L. Mito-Morphosis: Mitochondrial Fusion, Fission, and Cristae Remodeling as Key Mediators of Cellular Function. Annu. Rev. Physiol. 2016, 78, 505–531. [Google Scholar] [CrossRef]
- Cogliati, S.; Frezza, C.; Soriano, M.E.; Varanita, T.; Quintana-Cabrera, R.; Corrado, M.; Cipolat, S.; Costa, V.; Casarin, A.; Gomes, L.C.; et al. Mitochondrial Cristae Shape Determines Respiratory Chain Supercomplexes Assembly and Respiratory Efficiency. Cell 2013, 155, 160–171. [Google Scholar] [CrossRef] [Green Version]
- Lippi, G.; Mattiuzzi, C.; Cervellin, G. Critical review and meta-analysis on the combination of heart-type fatty acid binding protein (H-FABP) and troponin for early diagnosis of acute myocardial infarction. Clin. Biochem. 2013, 46, 26–30. [Google Scholar] [CrossRef]
- Ye, X.D.; He, Y.; Wang, S.; Wong, G.T.; Irwin, M.G.; Xia, Z. Heart-type fatty acid binding protein (H-FABP) as a biomarker for acute myocardial injury and long-term post-ischemic prognosis. Acta Pharmacol. Sin. 2018, 39, 1155–1163. [Google Scholar] [CrossRef]
- Maneechote, C.; Palee, S.; Chattipakorn, S.C.; Chattipakorn, N. Roles of mitochondrial dynamics modulators in cardiac ischaemia/reperfusion injury. J. Cell. Mol. Med. 2017, 21, 2643–2653. [Google Scholar] [CrossRef]
- Vasquez-Trincado, C.; Garcia-Carvajal, I.; Pennanen, C.; Parra, V.; Hill, J.A.; Rothermel, B.A.; Lavandero, S. Mitochondrial dynamics, mitophagy and cardiovascular disease. J. Physiol. 2016, 594, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Ritterhoff, J.; Tian, R. Metabolic mechanisms in physiological and pathological cardiac hypertrophy: New paradigms and challenges. Nat. Rev. Cardiol. 2023; ahead of print. [Google Scholar] [CrossRef]
- Eugenin, E.A. Role of Connexin/Pannexin containing channels in infectious diseases. FEBS Lett. 2014, 588, 1389–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Smet, M.A.J.; Lissoni, A.; Nezlobinsky, T.; Wang, N.; Dries, E.; Perez-Hernandez, M.; Lin, X.M.; Amoni, M.; Vervliet, T.; Witschas, K.; et al. Cx43 hemichannel microdomain signaling at the intercalated disc enhances cardiac excitability. J. Clin. Investig. 2021, 131, e137752. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kang, N.; Lovatt, D.; Torres, A.; Zhao, Z.; Lin, J.; Nedergaard, M. Connexin 43 hemichannels are permeable to ATP. J. Neurosci. 2008, 28, 4702–4711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, D.L.; Liu, X.Y.; Liu, R.J.; Yang, L.; Zuo, J.; Liu, W. Connexin 43 hemichannel regulates H9c2 cell proliferation by modulating intracellular ATP and Ca2+. Acta Biochim. Et Biophys. Sin. 2010, 42, 472–482. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.F.; Chen, X.L.; Lu, Y.L.; Fan, S.J.; Yang, Y.J.; Chen, Q.; Huang, Q.Y.; Xia, L.; Wei, Y.; Zheng, J.; et al. Diphenyleneiodonium enhances P2X7 dependent non-opsonized phagocytosis and suppresses inflammasome activation via blocking CX43-mediated ATP leakage. Pharmacol. Res. 2021, 166, 105470. [Google Scholar] [CrossRef]
- Dosch, M.; Zindel, J.; Jebbawi, F.; Melin, N.; Sanchez-Taltavull, D.; Stroka, D.; Candinas, D.; Beldi, G. Connexin-43-dependent ATP release mediates macrophage activation during sepsis. Elife 2019, 8, e42670. [Google Scholar] [CrossRef]
- Hoorelbeke, D.; Decrock, E.; De Smet, M.; De Bock, M.; Descamps, B.; Van Haver, V.; Delvaeye, T.; Krysko, D.V.; Vanhove, C.; Bultynck, G.; et al. Cx43 channels and signaling via IP(3)/Ca2+, ATP, and ROS/NO propagate radiation-induced DNA damage to non-irradiated brain microvascular endothelial cells. Cell Death Dis. 2020, 11, 194. [Google Scholar] [CrossRef] [Green Version]
- Elias, L.A.; Wang, D.D.; Kriegstein, A.R. Gap junction adhesion is necessary for radial migration in the neocortex. Nature 2007, 448, 901–907. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Xu, X.; Li, D.; Yao, B.; Wang, H.; Zhao, L.; Wang, H.; Dong, J.; Zhang, J.; Peng, R. Role of Cx43 in iPSC-CM Damage Induced by Microwave Radiation. Int. J. Mol. Sci. 2023, 24, 12533. https://doi.org/10.3390/ijms241612533
Yin Y, Xu X, Li D, Yao B, Wang H, Zhao L, Wang H, Dong J, Zhang J, Peng R. Role of Cx43 in iPSC-CM Damage Induced by Microwave Radiation. International Journal of Molecular Sciences. 2023; 24(16):12533. https://doi.org/10.3390/ijms241612533
Chicago/Turabian StyleYin, Yue, Xinping Xu, Dayan Li, Binwei Yao, Haoyu Wang, Li Zhao, Hui Wang, Ji Dong, Jing Zhang, and Ruiyun Peng. 2023. "Role of Cx43 in iPSC-CM Damage Induced by Microwave Radiation" International Journal of Molecular Sciences 24, no. 16: 12533. https://doi.org/10.3390/ijms241612533
APA StyleYin, Y., Xu, X., Li, D., Yao, B., Wang, H., Zhao, L., Wang, H., Dong, J., Zhang, J., & Peng, R. (2023). Role of Cx43 in iPSC-CM Damage Induced by Microwave Radiation. International Journal of Molecular Sciences, 24(16), 12533. https://doi.org/10.3390/ijms241612533