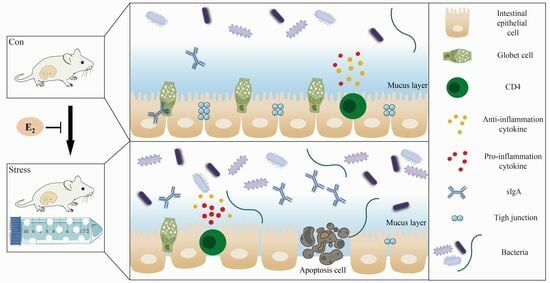
Chronic Stress That Changed Intestinal Permeability and Induced Inflammation Was Restored by Estrogen
Abstract
:1. Introduction
2. Results
2.1. Effect of Chronic Stress on Pregnant Mice
2.2. Chronic Stress Broke the Intestine Mucosal Mechanical Barrier
2.3. Chronic Stress Damaged Intestinal Mucus Layer of Pregnant Mice
2.4. Effect of Chronic Stress on Pregnant Mice Affected Intestinal Mucosal Immune Function
2.5. Effect of Chronic Stress on Pregnant Mice Altered Gut Microbiota Composition
2.6. Low Concentration E2 Protects Intestinal Epithelial Cells
3. Discussion
4. Materials and Methods
4.1. Animals and Treatment
4.2. HE and PAS Staining
4.3. Immunohistochemical Staining
4.4. Western Blotting
4.5. Measurement of Plasma NE, CORT, E2, sIgA, and Intestinal Inflammatory Factors Concentration
4.6. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
4.7. Cell Culture and Treatment
4.8. Cell Counting Kit-8 (CCK-8)
4.9. Microbial Sequencing
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becker, M.; Weinberger, T.; Chandy, A.; Schmukler, S. Depression during Pregnancy and Postpartum. Curr. Psychiatry Rep. 2016, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.L.; Taylor, N.F.; Shields, N.; Frawley, H.C. Attitudes, Barriers and Enablers to Physical Activity in Pregnant Women: A Systematic Review. J. Physiother. 2018, 64, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.M.; Melville, J.L.; Guo, Y.; Fan, M.-Y.; Gavin, A. Psychosocial Stress during Pregnancy. Am. J. Obstet. Gynecol. 2010, 202, 61.e1–61.e7. [Google Scholar] [CrossRef] [Green Version]
- Tomiyama, A.J. Stress and Obesity. Annu. Rev. Psychol. 2019, 70, 703–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagraauw, H.M.; Kuiper, J.; Bot, I. Acute and Chronic Psychological Stress as Risk Factors for Cardiovascular Disease: Insights Gained from Epidemiological, Clinical and Experimental Studies. Brain Behav. Immun. 2015, 50, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-S.; Wei, J.; Qin, L.; Kim, Y.; Yan, Z.; Greengard, P. Cellular and Molecular Basis for Stress-Induced Depression. Mol. Psychiatry 2017, 22, 1440–1447. [Google Scholar] [CrossRef] [Green Version]
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic Stress Promotes Tumor Growth and Angiogenesis in a Mouse Model of Ovarian Carcinoma. Nat. Med. 2006, 12, 939–944. [Google Scholar] [CrossRef]
- Dai, S.; Mo, Y.; Wang, Y.; Xiang, B.; Liao, Q.; Zhou, M.; Li, X.; Li, Y.; Xiong, W.; Li, G.; et al. Chronic Stress Promotes Cancer Development. Front. Oncol. 2020, 10, 1492. [Google Scholar] [CrossRef]
- Ilchmann-Diounou, H.; Menard, S. Psychological Stress, Intestinal Barrier Dysfunctions, and Autoimmune Disorders: An Overview. Front. Immunol. 2020, 11, 1823. [Google Scholar] [CrossRef]
- Hill, J.H.; Round, J.L. SnapShot: Microbiota Effects on Host Physiology. Cell 2021, 184, 2796. [Google Scholar] [CrossRef]
- Qin, H.-Y.; Cheng, C.-W.; Tang, X.-D.; Bian, Z.-X. Impact of Psychological Stress on Irritable Bowel Syndrome. World J. Gastroenterol. 2014, 20, 14126–14131. [Google Scholar] [CrossRef] [PubMed]
- Christian, L.M. Physiological Reactivity to Psychological Stress in Human Pregnancy: Current Knowledge and Future Directions. Prog. Neurobiol. 2012, 99, 106–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grippo, A.J. The Utility of Animal Models in Understanding Links between Psychosocial Processes and Cardiovascular Health. Soc. Personal. Psychol. Compass 2011, 5, 164–179. [Google Scholar] [CrossRef] [Green Version]
- Van den Bergh, B.R.H.; van den Heuvel, M.I.; Lahti, M.; Braeken, M.; de Rooij, S.R.; Entringer, S.; Hoyer, D.; Roseboom, T.; Räikkönen, K.; King, S.; et al. Prenatal Developmental Origins of Behavior and Mental Health: The Influence of Maternal Stress in Pregnancy. Neurosci. Biobehav. Rev. 2020, 117, 26–64. [Google Scholar] [CrossRef] [Green Version]
- Tuovinen, S.; Lahti-Pulkkinen, M.; Girchenko, P.; Heinonen, K.; Lahti, J.; Reynolds, R.M.; Hämäläinen, E.; Villa, P.M.; Kajantie, E.; Laivuori, H.; et al. Maternal Antenatal Stress and Mental and Behavioral Disorders in Their Children. J. Affect. Disord. 2021, 278, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Vannuccini, S.; Bocchi, C.; Severi, F.M.; Challis, J.R.; Petraglia, F. Endocrinology of Human Parturition. Ann. Endocrinol. 2016, 77, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Trenti, A.; Tedesco, S.; Boscaro, C.; Trevisi, L.; Bolego, C.; Cignarella, A. Estrogen, Angiogenesis, Immunity and Cell Metabolism: Solving the Puzzle. Int. J. Mol. Sci. 2018, 19, 859. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Qi, Q.-R.; Li, Y.; Day, R.; Makhoul, J.; Magness, R.R.; Chen, D.-B. Estrogen Receptors and Estrogen-Induced Uterine Vasodilation in Pregnancy. Int. J. Mol. Sci. 2020, 21, 4349. [Google Scholar] [CrossRef]
- Rosenfeld, C.R.; Rivera, R. Circulatory Responses to Systemic Infusions of Estrone and Estradiol-17alpha in Nonpregnant, Oophorectomized Ewes. Am. J. Obstet. Gynecol. 1978, 132, 442–448. [Google Scholar] [CrossRef]
- Prediger, M.E.; Gamaro, G.D.; Crema, L.M.; Fontella, F.U.; Dalmaz, C. Estradiol Protects against Oxidative Stress Induced by Chronic Variate Stress. Neurochem. Res. 2004, 29, 1923–1930. [Google Scholar] [CrossRef]
- Bellanti, F.; Matteo, M.; Rollo, T.; De Rosario, F.; Greco, P.; Vendemiale, G.; Serviddio, G. Sex Hormones Modulate Circulating Antioxidant Enzymes: Impact of Estrogen Therapy. Redox Biol. 2013, 1, 340–346. [Google Scholar] [CrossRef] [Green Version]
- Deroo, B.J.; Korach, K.S. Estrogen Receptors and Human Disease. J. Clin. Investig. 2006, 116, 561–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillies, G.E.; McArthur, S. Estrogen Actions in the Brain and the Basis for Differential Action in Men and Women: A Case for Sex-Specific Medicines. Pharmacol. Rev. 2010, 62, 155–198. [Google Scholar] [CrossRef] [Green Version]
- Wrzosek, L.; Miquel, S.; Noordine, M.-L.; Bouet, S.; Joncquel Chevalier-Curt, M.; Robert, V.; Philippe, C.; Bridonneau, C.; Cherbuy, C.; Robbe-Masselot, C.; et al. Bacteroides Thetaiotaomicron and Faecalibacterium Prausnitzii Influence the Production of Mucus Glycans and the Development of Goblet Cells in the Colonic Epithelium of a Gnotobiotic Model Rodent. BMC Biol. 2013, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- Bain, C.C.; Mowat, A.M. Macrophages in Intestinal Homeostasis and Inflammation. Immunol. Rev. 2014, 260, 102–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rorsman, B.; Gräsbeck, A.; Hagnell, O.; Lanke, J.; Ohman, R.; Ojesjö, L.; Otterbeck, L. A Prospective Study of First-Incidence Depression. The Lundby Study, 1957–1972. Br. J. Psychiatry 1990, 156, 336–342. [Google Scholar] [CrossRef]
- Kessler, R.C.; McGonagle, K.A.; Swartz, M.; Blazer, D.G.; Nelson, C.B. Sex and Depression in the National Comorbidity Survey. I: Lifetime Prevalence, Chronicity and Recurrence. J. Affect. Disord. 1993, 29, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Weissman, M.M.; Bland, R.C.; Canino, G.J.; Faravelli, C.; Greenwald, S.; Hwu, H.G.; Joyce, P.R.; Karam, E.G.; Lee, C.K.; Lellouch, J.; et al. Cross-National Epidemiology of Major Depression and Bipolar Disorder. JAMA 1996, 276, 293–299. [Google Scholar] [CrossRef]
- Christian, L.M. Psychoneuroimmunology in Pregnancy: Immune Pathways Linking Stress with Maternal Health, Adverse Birth Outcomes, and Fetal Development. Neurosci. Biobehav. Rev. 2012, 36, 350–361. [Google Scholar] [CrossRef] [Green Version]
- Procopio, M. Maternal Exposure to Death of a First Degree Relative during First Trimester of Pregnancy Increases Risk of Schizophrenia in Offspring. Evid. Based Ment. Health 2008, 11, 127. [Google Scholar] [CrossRef]
- Herba, C.M.; Glover, V.; Ramchandani, P.G.; Rondon, M.B. Maternal Depression and Mental Health in Early Childhood: An Examination of Underlying Mechanisms in Low-Income and Middle-Income Countries. Lancet Psychiatry 2016, 3, 983–992. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, W.; Li, Z.-Z.; Zhang, C.; Huang, C.; Yang, J.; Kong, G.-Y.; Li, Z.-F. Repetitive Restraint Stress Changes Spleen Immune Cell Subsets through Glucocorticoid Receptor or β-Adrenergic Receptor in a Stage Dependent Manner. Biochem. Biophys. Res. Commun. 2018, 495, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Viau, V.; Meaney, M.J. Variations in the Hypothalamic-Pituitary-Adrenal Response to Stress during the Estrous Cycle in the Rat. Endocrinology 1991, 129, 2503–2511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgess, L.H.; Handa, R.J. Chronic Estrogen-Induced Alterations in Adrenocorticotropin and Corticosterone Secretion, and Glucocorticoid Receptor-Mediated Functions in Female Rats. Endocrinology 1992, 131, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Carey, M.P.; Deterd, C.H.; de Koning, J.; Helmerhorst, F.; de Kloet, E.R. The Influence of Ovarian Steroids on Hypothalamic-Pituitary-Adrenal Regulation in the Female Rat. J. Endocrinol. 1995, 144, 311–321. [Google Scholar] [CrossRef]
- Redei, E.; Li, L.; Halasz, I.; McGivern, R.F.; Aird, F. Fast Glucocorticoid Feedback Inhibition of ACTH Secretion in the Ovariectomized Rat: Effect of Chronic Estrogen and Progesterone. Neuroendocrinology 1994, 60, 113–123. [Google Scholar] [CrossRef]
- Komesaroff, P.A.; Esler, M.; Clarke, I.J.; Fullerton, M.J.; Funder, J.W. Effects of Estrogen and Estrous Cycle on Glucocorticoid and Catecholamine Responses to Stress in Sheep. Am. J. Physiol. 1998, 275, E671–E678. [Google Scholar] [CrossRef]
- Dayas, C.V.; Xu, Y.; Buller, K.M.; Day, T.A. Effects of Chronic Oestrogen Replacement on Stress-Induced Activation of Hypothalamic-Pituitary-Adrenal Axis Control Pathways. J. Neuroendocrinol. 2000, 12, 784–794. [Google Scholar] [CrossRef]
- Young, E.A.; Altemus, M.; Parkison, V.; Shastry, S. Effects of Estrogen Antagonists and Agonists on the ACTH Response to Restraint Stress in Female Rats. Neuropsychopharmacology 2001, 25, 881–891. [Google Scholar] [CrossRef]
- Schmidt, P.J.; Rubinow, D.R. Sex Hormones and Mood in the Perimenopause. Ann. N. Y Acad. Sci. 2009, 1179, 70–85. [Google Scholar] [CrossRef] [Green Version]
- Söderholm, J.D.; Yates, D.A.; Gareau, M.G.; Yang, P.-C.; MacQueen, G.; Perdue, M.H. Neonatal Maternal Separation Predisposes Adult Rats to Colonic Barrier Dysfunction in Response to Mild Stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G1257–G1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Yin, J.; Du, M.; Yan, P.; Xu, J.; Zhu, X.; Yu, J. Heat-Stress-Induced Damage to Porcine Small Intestinal Epithelium Associated with Downregulation of Epithelial Growth Factor Signaling. J. Anim. Sci. 2009, 87, 1941–1949. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Yin, P.; Liu, F.; Cheng, G.; Guo, K.; Lu, A.; Zhu, X.; Luan, W.; Xu, J. Effect of Heat Stress on the Porcine Small Intestine: A Morphological and Gene Expression Study. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 156, 119–128. [Google Scholar] [CrossRef]
- George, S.K.; Jiao, Y.; Bishop, C.E.; Lu, B. Oxidative Stress Is Involved in Age-Dependent Spermatogenic Damage of Immp2l Mutant Mice. Free Radic. Biol. Med. 2012, 52, 2223–2233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micale, N.; Vairagoundar, R.; Yakovlev, A.G.; Kozikowski, A.P. Design and Synthesis of a Potent and Selective Peptidomimetic Inhibitor of Caspase-3. J. Med. Chem. 2004, 47, 6455–6458. [Google Scholar] [CrossRef]
- Suresh, K.; Carino, K.; Johnston, L.; Servinsky, L.; Machamer, C.E.; Kolb, T.M.; Lam, H.; Dudek, S.M.; An, S.S.; Rane, M.J.; et al. A Nonapoptotic Endothelial Barrier-Protective Role for Caspase-3. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L1118–L1126. [Google Scholar] [CrossRef] [PubMed]
- Strzalka, W.; Ziemienowicz, A. Proliferating Cell Nuclear Antigen (PCNA): A Key Factor in DNA Replication and Cell Cycle Regulation. Ann. Bot. 2011, 107, 1127–1140. [Google Scholar] [CrossRef] [Green Version]
- Er, H.; Acar, N.; Kipmen-Korgun, D.; Celik-Ozenci, C.; Ustunel, I.; Asar, M.; Korgun, E.T. Determination of PCNA, Cyclin D3, P27, P57 and Apoptosis Rate in Normal and Dexamethasone-Induced Intrauterine Growth Restricted Rat Placentas. Acta Histochem. 2015, 117, 137–147. [Google Scholar] [CrossRef]
- Shyu, K.G.; Kuan, P.; Chang, M.L.; Wang, B.W.; Huang, F.Y. Effects of Norepinephrine on Apoptosis in Rat Neonatal Cardiomyocytes. J. Formos. Med. Assoc. 2000, 99, 412–418. [Google Scholar]
- Gao, H.-B.; Tong, M.-H.; Hu, Y.-Q.; Guo, Q.-S.; Ge, R.; Hardy, M.P. Glucocorticoid Induces Apoptosis in Rat Leydig Cells. Endocrinology 2002, 143, 130–138. [Google Scholar] [CrossRef]
- Ma, Y.-X.; Guo, Z.; Sun, T. CGRP Inhibits Norepinephrine Induced Apoptosis with Restoration of Bcl-2/Bax in Cultured Cardiomyocytes of Rat. Neurosci. Lett. 2013, 549, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Sánchez de Medina, F.; Romero-Calvo, I.; Mascaraque, C.; Martínez-Augustin, O. Intestinal Inflammation and Mucosal Barrier Function. Inflamm. Bowel Dis. 2014, 20, 2394–2404. [Google Scholar] [CrossRef] [PubMed]
- Lepage, P.; Seksik, P.; Sutren, M.; de la Cochetière, M.-F.; Jian, R.; Marteau, P.; Doré, J. Biodiversity of the Mucosa-Associated Microbiota Is Stable along the Distal Digestive Tract in Healthy Individuals and Patients with IBD. Inflamm. Bowel Dis. 2005, 11, 473–480. [Google Scholar] [CrossRef]
- Mowat, A.M.; Agace, W.W. Regional Specialization within the Intestinal Immune System. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Padro, C.J.; Sanders, V.M. Neuroendocrine Regulation of Inflammation. Semin. Immunol. 2014, 26, 357–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhabhar, F.S. The Short-Term Stress Response—Mother Nature’s Mechanism for Enhancing Protection and Performance under Conditions of Threat, Challenge, and Opportunity. Front. Neuroendocrinol. 2018, 49, 175–192. [Google Scholar] [CrossRef]
- Söderholm, J.D.; Yang, P.-C.; Ceponis, P.; Vohra, A.; Riddell, R.; Sherman, P.M.; Perdue, M.H. Chronic Stress Induces Mast Cell-Dependent Bacterial Adherence and Initiates Mucosal Inflammation in Rat Intestine. Gastroenterology 2002, 123, 1099–1108. [Google Scholar] [CrossRef]
- Poroyko, V.A.; Carreras, A.; Khalyfa, A.; Khalyfa, A.A.; Leone, V.; Peris, E.; Almendros, I.; Gileles-Hillel, A.; Qiao, Z.; Hubert, N.; et al. Chronic Sleep Disruption Alters Gut Microbiota, Induces Systemic and Adipose Tissue Inflammation and Insulin Resistance in Mice. Sci. Rep. 2016, 6, 35405. [Google Scholar] [CrossRef] [Green Version]
- Sears, C.L. A Dynamic Partnership: Celebrating Our Gut Flora. Anaerobe 2005, 11, 247–251. [Google Scholar] [CrossRef]
- Wexler, H.M. Bacteroides: The Good, the Bad, and the Nitty-Gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef] [Green Version]
- Mirpuri, J.; Raetz, M.; Sturge, C.R.; Wilhelm, C.L.; Benson, A.; Savani, R.C.; Hooper, L.V.; Yarovinsky, F. Proteobacteria-Specific IgA Regulates Maturation of the Intestinal Microbiota. Gut Microbes 2014, 5, 28–39. [Google Scholar] [CrossRef] [Green Version]
- Neu, J.; Walker, W.A. Necrotizing Enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Bury, R.G.; Tudehope, D. Enteral Antibiotics for Preventing Necrotizing Enterocolitis in Low Birthweight or Preterm Infants. Cochrane Database Syst. Rev. 2001, CD000405. [Google Scholar] [CrossRef] [PubMed]
- Beiranvand, S.; Noparast, M.; Eslamizade, N.; Saeedikia, S. The Effects of Religion and Spirituality on Postoperative Pain, Hemodynamic Functioning and Anxiety after Cesarean Section. Acta Med. Iran. 2014, 52, 909–915. [Google Scholar] [PubMed]
- Tengstrand, B.; Carlström, K.; Felländer-Tsai, L.; Hafström, I. Abnormal Levels of Serum Dehydroepiandrosterone, Estrone, and Estradiol in Men with Rheumatoid Arthritis: High Correlation between Serum Estradiol and Current Degree of Inflammation. J. Rheumatol. 2003, 30, 2338–2343. [Google Scholar]
- Hudson, M.; Thombs, B.; Baron, M. Canadian Scleroderma Research Group Time to Diagnosis in Systemic Sclerosis: Is Sex a Factor? Arthritis Rheum. 2009, 61, 274–278. [Google Scholar] [CrossRef]
- Cutolo, M.; Wilder, R.L. Different Roles for Androgens and Estrogens in the Susceptibility to Autoimmune Rheumatic Diseases. Rheum. Dis. Clin. N. Am. 2000, 26, 825–839. [Google Scholar] [CrossRef]
- Cutolo, M.; Capellino, S.; Montagna, P.; Ghiorzo, P.; Sulli, A.; Villaggio, B. Sex Hormone Modulation of Cell Growth and Apoptosis of the Human Monocytic/Macrophage Cell Line. Arthritis Res. Ther. 2005, 7, R1124–R1132. [Google Scholar] [CrossRef] [Green Version]
- Denney, J.M.; Nelson, E.L.; Wadhwa, P.D.; Waters, T.P.; Mathew, L.; Chung, E.K.; Goldenberg, R.L.; Culhane, J.F. Longitudinal Modulation of Immune System Cytokine Profile during Pregnancy. Cytokine 2011, 53, 170–177. [Google Scholar] [CrossRef] [Green Version]








| Gene | Forward | Reverse |
|---|---|---|
| Muc-2 | 5′-CTGACCAAGAGCGAACACAA-3′ | 5′-CATGACTGGAAGCAACTGGA-3′ |
| Claudin-1 | 5′-AGGTCTGGCGACATTAGTGG-3′ | 5′-TGGTGTTGGGTAAGAGGTTG-3′ |
| Occludin | 5′-CTTTGGCTACGGAGGTGGCTAT-3′ | 5′-CTTTGGCTGCTCTTGGGTCTG-3′ |
| ZO-1 | 5′-GCATGTAGACCCAGCAAAGG-3′ | 5′-GGTTTTGTCTCATCATTTCCTCA-3′ |
| β-actin | 5′-TGCTGTCCCTGTATGCCTCTG-3′ | 5′-TTGATGTCACGCACGATTTCC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wan, H.; Ma, R.; Liu, T.; Chen, Y.; Dong, Y. Chronic Stress That Changed Intestinal Permeability and Induced Inflammation Was Restored by Estrogen. Int. J. Mol. Sci. 2023, 24, 12822. https://doi.org/10.3390/ijms241612822
Li Y, Wan H, Ma R, Liu T, Chen Y, Dong Y. Chronic Stress That Changed Intestinal Permeability and Induced Inflammation Was Restored by Estrogen. International Journal of Molecular Sciences. 2023; 24(16):12822. https://doi.org/10.3390/ijms241612822
Chicago/Turabian StyleLi, Yuanyuan, Huayun Wan, Ruiqin Ma, Tianya Liu, Yaoxing Chen, and Yulan Dong. 2023. "Chronic Stress That Changed Intestinal Permeability and Induced Inflammation Was Restored by Estrogen" International Journal of Molecular Sciences 24, no. 16: 12822. https://doi.org/10.3390/ijms241612822
APA StyleLi, Y., Wan, H., Ma, R., Liu, T., Chen, Y., & Dong, Y. (2023). Chronic Stress That Changed Intestinal Permeability and Induced Inflammation Was Restored by Estrogen. International Journal of Molecular Sciences, 24(16), 12822. https://doi.org/10.3390/ijms241612822