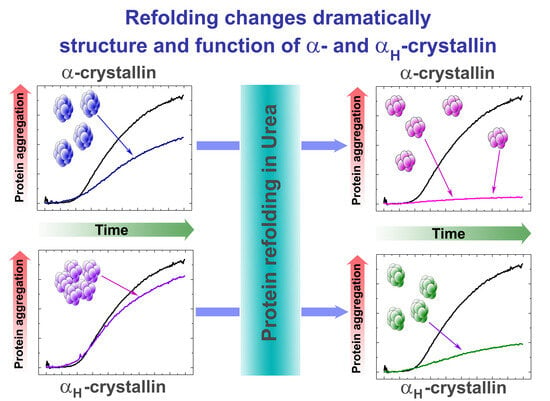
Refolding Increases the Chaperone-like Activity of αH-Crystallin and Reduces Its Hydrodynamic Diameter to That of α-Crystallin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Refolding of α-Crystallin and HMWA
2.1.1. Study of a Size of α-Crystallin and HMWA Particles under Urea-Induced Denaturation and Renaturation
2.1.2. Study of Thermostability of Native and Refolded α-Crystallin and HMWA by DSC
2.2. Investigation of the Chaperone-like Activity of HMWA in Comparison with α-Crystallin
2.2.1. Kinetics of UV-Damaged βL-Crystallin Aggregation
2.2.2. Chaperone-like Activity of Native and Refolded α-Crystallin and HMWA
3. Materials and Methods
3.1. Materials
3.2. Isolation and Purification of Crystallin
3.3. Denaturation and Renaturation of α-Crystallin and HMWA
3.4. Dynamic Light Scattering
3.5. Size Exclusion Chromatography
3.6. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
3.7. Calorimetric Studies
3.8. UV Irradiation of βL-Crystallin Preparation
3.9. Optical Density of Incubation Medium and the Amount of Aggregated Protein
3.10. Study of the Aggregation Kinetics of UV-Damaged βL-Crystallin
- A—apparent optical density of the solution
- t—time
- t0—the duration of the nucleation stage
- υ0—the initial rate of the visible stage of aggregation
- B—constant
3.11. Measurement of the Chaperone-like Activity of α-Crystallin and HMWA
3.12. Statistic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fricke, T.; Holden, B.; Wilson, D.; Schlenther, G.; Naidoo, K.; Resnikoff, S.; Frick, K. Global cost of correcting vision impairment from uncorrected refractive error. Bull. World Health Organ. 2012, 90, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Resnikoff, S. Global magnitude of visual impairment caused by uncorrected refractive errors in 2004. Bull. World Health Organ. 2008, 86, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Benedek, G.B. Theory of Transparency of the Eye. Appl. Opt. 1971, 10, 459–473. [Google Scholar] [CrossRef]
- Costello, M.J.; Burette, A.; Weber, M.; Metlapally, S.; Gilliland, K.O.; Fowler, W.C.; Mohamed, A.; Johnsen, S. Electron tomography of fiber cell cytoplasm and dense cores of multilamellar bodies from human age-related nuclear cataracts. Exp. Eye Res. 2012, 101, 72–81. [Google Scholar] [CrossRef]
- Costello, M.J.; Johnsen, S.; Metlapally, S.; Gilliland, K.O.; Frame, L.; Balasubramanian, D. Multilamellar spherical particles as potential sources of excessive light scattering in human age-related nuclear cataracts. Exp. Eye Res. 2010, 91, 881–889. [Google Scholar] [CrossRef]
- Bloemendal, H. The Vertebrate Eye Lens. Science 1977, 197, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Bloemendal, H.; de Jong, W.; Jaenicke, R.; Lubsen, N.H.; Slingsby, C.; Tardieu, A. Ageing and vision: Structure, stability and function of lens crystallins. Prog. Biophys. Mol. Biol. 2004, 86, 407–485. [Google Scholar] [CrossRef]
- Horwitz, J. Alpha-crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. USA 1992, 89, 10449–10453. [Google Scholar] [CrossRef]
- Rao, P.V.; Huang, Q.L.; Horwitz, J.; Zigler, J. Evidence that α-crystallin prevents non-specific protein aggregation in the intact eye lens. Biochim. Biophys. Acta 1995, 1245, 439–447. [Google Scholar] [CrossRef]
- Haslbeck, M.; Weinkauf, S.; Buchner, J. Small heat shock proteins: Simplicity meets complexity. J. Biol. Chem. 2019, 294, 2121–2132. [Google Scholar] [CrossRef]
- Haslbeck, M.; Peschek, J.; Buchner, J.; Weinkauf, S. Structure and function of alpha-crystallins: Traversing from in vitro to in vivo. Biochim. Biophys. Acta 2016, 1860, 149–166. [Google Scholar] [PubMed]
- Muranov, K.O.; Ostrovsky, M.A. Biochemistry of eye lens in the norm and in cataractogenesis. Biochemistry 2022, 87, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Kenworthy, A.K.; Magid, A.D.; Oliver, T.N.; McIntosh, T.J. Colloid osmotic pressure of steer and β-crystallins: Possible functional roles for lens crystallin distribution and structural diversity. Exp. Eye Res. 1994, 59, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Kramps, H.A.; Stols, A.L.H.; Hoenders, H.J. On the quaternary structure of high-molecular-weight proteins from the bovine eye lens. J. Biol. Inorg. Chem. 1975, 50, 503–509. [Google Scholar] [CrossRef]
- Harding, J.J.; Dilley, K.J. Structural proteins of the mammalian lens: A review with emphasis on changes in development, aging and cataract. Exp. Eye Res. 1976, 22, 1–73. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.; Freund, T.; Li, L.K.; Augusteyn, R.C. Age-dependent changes in the structure of alpha crystallin. Investig. Ophthalmol. Vis. Sci. 1971, 10, 677–686. [Google Scholar]
- Srivastava, O.P.; Srivastava, K.; Silney, C. Levels of crystallin fragments and identification of their origin in water soluble high molecular weight (HMW) proteins of human lenses. Curr. Eye Res. 1996, 15, 511–520. [Google Scholar] [CrossRef]
- Krivandin, A.V.; Muranov, K.O.; Yakovlev, F.Y.; Poliansky, N.B.; Wasserman, L.A.; Ostrovsky, M.A. Resistance of α-crystallin quaternary structure to UV irradiation. Biochemistry 2009, 74, 633–642. [Google Scholar] [CrossRef]
- A Padgaonkar, V.; Leverenz, V.R.; Fowler, K.E.; Reddy, V.N.; Giblin, F.J. The effects of hyperbaric oxygen on the crystallins of cultured rabbit lenses: A possible catalytic role for copper. Exp. Eye Res. 2000, 71, 371–383. [Google Scholar] [CrossRef]
- Chiou, S.H.; Azari, P. Physicochemical characterization of α-crystallins from bovine lenses: Hydrodynamic and conformational properties. Protein J. 1989, 8, 1–17. [Google Scholar] [CrossRef]
- Liang, J.; Rossi, M. Near-ultraviolet circular dichroism of bovine high molecular weight alpha-crystallin. Investig. Ophthalmol. Vis. Sci. 1989, 30, 2065–2068. [Google Scholar]
- Liang, J.; Bose, S.; Chakrabarti, B. Age-related changes in protein conformation in bovine lens crystallins. Exp. Eye Res. 1985, 40, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Messmer, M.; Chakrabarti, B. High-molecular-weight protein aggregates of calf and cow lens: Spectroscopic evaluation. Exp. Eye Res. 1988, 47, 173–183. [Google Scholar] [CrossRef]
- Carver, J.A.; Nicholls, K.A.; Aquilina, A.J.; Truscott, R.J. Age-related changes in bovine α-crystallin and high-molecular-weight protein. Exp. Eye Res. 1996, 63, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, L.; Boyle, D. Molecular chaperone properties of the high molecular weight aggregate from aged lens. Curr. Eye Res. 1994, 13, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.J.; Akhtar, N.J. Human Lens High-Molecular-Weight α-Crystallin Aggregates. Biochem. Biophys. Res. Commun. 2000, 275, 354–359. [Google Scholar] [CrossRef]
- Huang, Q.; Ding, L.; Phan, K.B.; Cheng, C.; Xia, C.-H.; Gong, X.; Horwitz, J. Mechanism of cataract formation in αA-crystallin Y118D mutation. Investig. Opthalmol. Vis. Sci. 2009, 50, 2919–2926. [Google Scholar] [CrossRef]
- Xia, C.H.; Liu, H.; Chang, B.; Cheng, C.; Cheung, D.; Wang, M.; Huang, Q.; Horwitz, J.; Gong, X. Arginine 54 and tyrosine 118 residues of αA-crystallin are crucial for lens formation and transparency. Investig. Opthalmol. Vis. Sci. 2006, 47, 3004–3010. [Google Scholar] [CrossRef]
- Saha, S.; Das, K.P. Unfolding and refolding of bovine α-crystallin in urea and its chaperone activity. Protein J. 2007, 26, 315–326. [Google Scholar] [CrossRef]
- Siezen, R.J.; Bindels, J.G. Stepwise dissociation/denaturation and reassociation/renaturation of bovine α-crystallin in urea and guanidine hydrochloride: Sedimentation, fluorescence, near-ultraviolet and far-ultraviolet circular dichroism studies. Exp. Eye Res. 1982, 34, 969–983. [Google Scholar] [CrossRef]
- Borzova, V.A.; Eronina, T.B.; Mikhaylova, V.V.; Roman, S.G.; Chernikov, A.M.; Chebotareva, N.A. Effect of Chemical Chaperones on the Stability of Proteins during Heat– or Freeze–Thaw Stress. Int. J. Mol. Sci. 2023, 24, 10298. [Google Scholar] [CrossRef]
- Eronina, T.B.; Mikhaylova, V.V.; Chebotareva, N.A.; Kurganov, B.I. Kinetic regime of thermal aggregation of holo- and apoglycogen phosphorylases b. Int. J. Biol. Macromol. 2016, 92, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Kurganov, B.I. Quantification of anti-aggregation activity of chaperones. Int. J. Biol. Macromol. 2017, 100, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylova, V.V.; Eronina, T.B.; Chebotareva, N.A.; Kurganov, B.I. The Effect of Chemical Chaperones on Proteins with Different Aggregation Kinetics. Biochemistry 2023, 88, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylova, V.V.; Eronina, T.B.; Chebotareva, N.A.; Kleymenov, S.Y.; Shubin, V.V.; Kurganov, B.I. A thermal after-effect of UV irradiation of muscle glycogen phosphorylase b. PLoS ONE 2017, 12, e0189125. [Google Scholar] [CrossRef] [PubMed]
- Muranov, K.; Poliansky, N.; Chebotareva, N.; Kleimenov, S.; Bugrova, A.; Indeykina, M.; Kononikhin, A.; Nikolaev, E.; Ostrovsky, M. The mechanism of the interaction of α-crystallin and UV-damaged βL-crystallin. Int. J. Biol. Macromol. 2019, 140, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Muranov, K.O.; Maloletkina, O.I.; Poliansky, N.B.; Markossian, K.A.; Kleimenov, S.Y.; Rozhkov, S.P.; Goryunov, A.S.; Ostrovsky, M.A.; Kurganov, B.I. Mechanism of aggregation of UV-irradiated betaL-crystallin. Exp. Eye Res. 2011, 92, 76–86. [Google Scholar]
- Augusteyn, R. α-Crystallin polymers and polymerization: The view from down under. Int. J. Biol. Macromol. 1998, 22, 253–262. [Google Scholar] [CrossRef]
- Chowdhury, A.; Choudhury, A.; Banerjee, V.; Banerjee, R.; Das, K.P. Spectroscopic studies of the unfolding of a multimeric protein α-crystallin. Biopolymers 2014, 101, 549–560. [Google Scholar] [CrossRef]
- Raman, B.; Ramakrishna, T.; Rao, C.M. Rapid refolding studies on the chaperone-like alpha-crystallin. Effect of al-pha-crystallin on refolding of beta- and gamma-crystallins. J. Biol. Chem. 1995, 270, 19888–19892. [Google Scholar]
- Borzova, V.A.; Markossian, K.A.; Muranov, K.O.; Polyansky, N.B.; Kleymenov, S.Y.; Kurganov, B.I. Quantification of anti-aggregation activity of UV-irradiated α-crystallin. Int. J. Biol. Macromol. 2015, 73, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Kurganov, B.I. Thermal after-effects of UV-irradiated lactate dehydrogenase from rabbit muscles. Biofizika 1965, 10, 875–876. [Google Scholar] [PubMed]
- Maloletkina, O.I.; Markossian, K.A.; Chebotareva, N.A.; Asryants, R.A.; Kleymenov, S.Y.; Poliansky, N.B.; Muranov, K.O.; Makeeva, V.F.; Kurganov, B.I. Kinetics of aggregation of UV-irradiated glyceraldehyde-3-phosphate dehydrogenase from rabbit skeletal muscle. Effect of agents possessing chaperone-like activity. Biophys. Chem. 2012, 163–164, 11–20. [Google Scholar] [CrossRef]
- Eronina, T.B.; Mikhaylova, V.V.; Chebotareva, N.A.; Makeeva, V.F.; Kurganov, B.I. Checking for reversibility of aggregation of UV-irradiated glycogen phosphorylase b under crowding conditions. Int. J. Biol. Macromol. 2016, 86, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Kurganov, B.I. Kinetic regime of aggregation of UV-irradiated glyceraldehyde-3-phosphate dehydrogenase from rabbit skeletal muscle. Biochem. Biophys. Res. Commun. 2018, 495, 1182–1186. [Google Scholar] [CrossRef]
- van Boekel, M.A.J.S. Multiresponse Kinetic Modeling of Chemical Reactions, Kinetic Modeling of Reactions in Foods; CRC Press: Boca Raton, FL, USA, 2008; p. 16.e28. [Google Scholar] [CrossRef]
- Kurganov, B.I.; Rafikova, E.R.; Dobrov, E.N. Kinetics of thermal aggregation of tobacco mosaic virus coat protein. Biochemistry 2002, 67, 525–533. [Google Scholar] [CrossRef]
- Wang, K.; I Kurganov, B. Kinetics of heat- and acidification-induced aggregation of firefly luciferase. Biophys. Chem. 2003, 106, 97–109. [Google Scholar] [CrossRef]
- Fedurkina, N.V.; Belousova, L.V.; Mitskevich, L.G.; Zhou, H.-M.; Chang, Z.; Kurganov, B.I. Change in kinetic regime of protein aggregation with temperature increase. Thermal aggregation of rabbit muscle creatine kinase. Biochemistry 2006, 71, 325–331. [Google Scholar] [CrossRef]
- Eronina, T.B.; Chebotareva, N.A.; Kurganov, B.I. Influence of osmolytes on inactivation and aggregation of muscle glycogen phosphorylase b by guanidine hydrochloride. stimulation of protein aggregation under crowding conditions. Biochemistry 2005, 70, 1020–1026. [Google Scholar] [CrossRef]
- Kurganov, B.I. Protein Aggregation Kinetics. In Chemical and Biological Kinetics; Burlakova, E.B., Varfolomeev, S.D., Eds.; Koninklijke Brill NV: Leiden, The Netherlands, 2005. [Google Scholar]
- Kurganov, B.I. Kinetics of protein aggregation. Quantitative estimation of the chaperone-like activity in test-systems based on suppression of protein aggregation. Biochemistry 2002, 67, 409–422. [Google Scholar] [CrossRef]
- Eronina, T.B.; Mikhaylova, V.V.; Chebotareva, N.A.; Borzova, V.A.; Yudin, I.K.; Kurganov, B.I. Mechanism of aggregation of UV-irradiated glycogen phosphorylase b at a low temperature in the presence of crowders and trimethylamine N-oxide. Biophys. Chem. 2018, 232, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Borzova, V.A.; Markossian, K.A.; Kara, D.A.; Chebotareva, N.A.; Makeeva, V.F.; Poliansky, N.B.; Muranov, K.O.; Kurganov, B.I. Quantification of anti-aggregation activity of chaperones: A test-system based on dithiothreitol-induced aggregation of bovine serum albumin. PLoS ONE 2013, 8, e74367. [Google Scholar] [CrossRef]
- Mikhaylova, V.V.; Eronina, T.B.; Chebotareva, N.A.; Shubin, V.V.; Kalacheva, D.I.; Kurganov, B.I. Effect of Arginine on Chaperone-Like Activity of HspB6 and Monomeric 14-3-3ζ. Int. J. Mol. Sci. 2020, 21, 2039. [Google Scholar] [CrossRef]
- Muranov, K.O.; Dizhevskaia, A.K.; Poliansky, N.B.; Dodonova, S.O.; Ostrovsky, M.A. Short-chain peptides as a promising class of chaperone-like anticataract agents: Molecular mechanism of inhibition of crystallin aggregation by pantethine. Russ. Chem. Bull. 2010, 59, 225–231. [Google Scholar] [CrossRef]
- Amm, I.; Sommer, T.; Wolf, D.H. Protein quality control and elimination of protein waste: The role of the ubiquitin–proteasome system. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2014, 1843, 182–196. [Google Scholar] [CrossRef]
- Powers, E.T.; Balch, W.E. Costly Mistakes: Translational Infidelity and Protein Homeostasis. Cell 2008, 134, 204–206. [Google Scholar] [CrossRef]
- Ganea, E.; Harding, J.J. Alpha-crystallin assists the renaturation of glyceraldehyde-3-phosphate dehydrogenase. Biochem. J. 2000, 345, 467–472. [Google Scholar] [CrossRef]
- Nath, D.; Rawat, U.; Anish, R.; Rao, M. α-Crystallin and ATP facilitate the in vitro renaturation of xylanase: Enhancement of refolding by metal ions. Protein Sci. 2002, 11, 2727–2734. [Google Scholar] [CrossRef]
- Chiou, S.H.; Azari, P.; Himmel, M.E.; Squire, P.G. Isolation and physical characterization of bovine lens crystallins. Int. J. Pept. Protein Res. 1979, 13, 409–417. [Google Scholar] [CrossRef]
- Putilina, T.; Skouri-Panet, F.; Prat, K.; Lubsen, N.H.; Tardieu, A. Subunit exchange demonstrates a differential chaperone activity of calf α-Crystallin toward βLOW- and Individual γ-Crystallins. J. Biol. Chem. 2003, 278, 13747–13756. [Google Scholar] [CrossRef]
- Itzhaki, R.F.; Gill, D. A micro-biuret method for estimating proteins. Anal. Biochem. 1964, 9, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Zetasizer. Zetasizer Nano User Manual; Malvern Instruments Ltd.: Malvern, UK, 2013. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- SPECTROstar® Nano, Manual. 2023. Available online: https://Brochures.Bmglabtech.Com/View/493641629/ (accessed on 18 July 2023).
- Eronina, T.B.; Chebotareva, N.A.; Roman, S.G.; Kleymenov, S.Y.; Makeeva, V.F.; Poliansky, N.B.; Muranov, K.O.; Kurganov, B.I. Thermal denaturation and aggregation of apoform of glycogen phosphorylaseb. Effect of crowding agents and chaperones. Biopolymers 2014, 101, 504–516. [Google Scholar] [CrossRef] [PubMed]








| α-Crystallin | HMWA | |||
|---|---|---|---|---|
| Native | Renatured | Native | Renatured | |
| Tmax, °C | 63.0 | 62.7 | 62.2 | 60.9 |
| Denaturation enthalpy, kJ∙Mol−1 | 240.1 | 338.8 | 167.1 | 124.2 |
| α-Crystallin | HMWA | |||
|---|---|---|---|---|
| Abscissa Point of Intersection | Target Protein/Chaperone mol/mol Ratio | Abscissa Point of Intersection | Target Protein/Chaperone mol/mol Ratio | |
| Native | 0.21 ± 0.02 1,2 | 5:1 | 0.46 ± 0.02 1,2 | 2:1 |
| Refolded | 0.08 ± 0.001 3 | 13:1 | 0.38 ± 0.01 3 | 3:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muranov, K.O.; Poliansky, N.B.; Borzova, V.A.; Kleimenov, S.Y. Refolding Increases the Chaperone-like Activity of αH-Crystallin and Reduces Its Hydrodynamic Diameter to That of α-Crystallin. Int. J. Mol. Sci. 2023, 24, 13473. https://doi.org/10.3390/ijms241713473
Muranov KO, Poliansky NB, Borzova VA, Kleimenov SY. Refolding Increases the Chaperone-like Activity of αH-Crystallin and Reduces Its Hydrodynamic Diameter to That of α-Crystallin. International Journal of Molecular Sciences. 2023; 24(17):13473. https://doi.org/10.3390/ijms241713473
Chicago/Turabian StyleMuranov, Konstantin O., Nicolay B. Poliansky, Vera A. Borzova, and Sergey Y. Kleimenov. 2023. "Refolding Increases the Chaperone-like Activity of αH-Crystallin and Reduces Its Hydrodynamic Diameter to That of α-Crystallin" International Journal of Molecular Sciences 24, no. 17: 13473. https://doi.org/10.3390/ijms241713473
APA StyleMuranov, K. O., Poliansky, N. B., Borzova, V. A., & Kleimenov, S. Y. (2023). Refolding Increases the Chaperone-like Activity of αH-Crystallin and Reduces Its Hydrodynamic Diameter to That of α-Crystallin. International Journal of Molecular Sciences, 24(17), 13473. https://doi.org/10.3390/ijms241713473