Neurofilament Light Chain Protein in Plasma and Extracellular Vesicles Is Associated with Minimal Hepatic Encephalopathy and Responses to Rifaximin Treatment in Cirrhotic Patients
Abstract
:1. Introduction
2. Results
2.1. Study Population
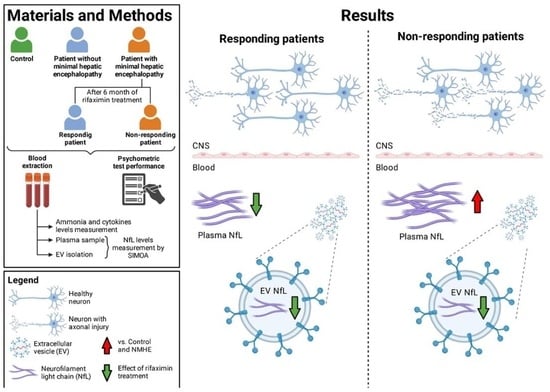
2.2. NfL Levels in Plasma and EVs
2.3. Correlation of Psychometric Tests and Inflammatory Parameters with NfL Levels in Plasma and EVs in Cirrhotic Patients
2.4. Correlation Analysis with PHES Score and Potential Interplay between Hyperammonemia and Inflammation on Plasma NfL Levels
2.5. Logistic Regression Analyses of Predictors of MHE Presence in Patients with Liver Cirrhosis
3. Discussion
4. Materials and Methods
4.1. Patients and Controls
4.2. Diagnosis of MHE, Psychometric Tests, and Rifaximin Treatment
4.3. Laboratory Measurements in Blood Samples
4.4. EV Isolation
4.5. NFL Measurement in Plasma and EV Samples
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nardone, R.; Taylor, A.C.; Höller, Y.; Brigo, F.; Lochner, P.; Trinka, E. Minimal Hepatic Encephalopathy: A Review. Neurosci. Res. 2016, 111, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ridola, L.; Nardelli, S.; Gioia, S.; Riggio, O. Quality of Life in Patients with Minimal Hepatic Encephalopathy. World J. Gastroenterol. 2018, 24, 5446–5453. [Google Scholar] [CrossRef] [PubMed]
- Weissenborn, K.; Ennen, J.C.; Schomerus, H.; Rückert, N.; Hecker, H. Neuropsychological Characterization of Hepatic Encephalopathy. J. Hepatol. 2001, 34, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Vilstrup, H.; Amodio, P.; Bajaj, J.; Cordoba, J.; Ferenci, P.; Mullen, K.D.; Weissenborn, K.; Wong, P. Hepatic Encephalopathy in Chronic Liver Disease: 2014 Practice Guideline by the American Association for the Study Of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014, 60, 715–735. [Google Scholar] [CrossRef] [PubMed]
- Montagnese, S.; Rautou, P.-E.; Romero-Gómez, M.; Larsen, F.S.; Shawcross, D.L.; Thabut, D.; Vilstrup, H.; Weissenborn, K. EASL Clinical Practice Guidelines on the Management of Hepatic Encephalopathy. J. Hepatol. 2022, 77, 807–824. [Google Scholar] [CrossRef]
- Mangas-Losada, A.; García-García, R.; Urios, A.; Escudero-García, D.; Tosca, J.; Giner-Durán, R.; Serra, M.A.; Montoliu, C.; Felipo, V. Minimal Hepatic Encephalopathy Is Associated with Expansion and Activation of CD4+CD28−, Th22 and Tfh and B Lymphocytes. Sci. Rep. 2017, 7, 6683. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Cao, B.; Tian, Q. Effects of SIBO and Rifaximin Therapy on MHE Caused by Hepatic Cirrhosis. Int. J. Clin. Exp. Med. 2015, 8, 2954–2957. [Google Scholar]
- Sidhu, S.S.; Goyal, O.; Mishra, B.P.; Sood, A.; Chhina, R.S.; Soni, R.K. Rifaximin Improves Psychometric Performance and Health-Related Quality of Life in Patients With Minimal Hepatic Encephalopathy (The RIME Trial). Am. J. Gastroenterol. 2011, 106, 307–316. [Google Scholar] [CrossRef]
- Mangas-Losada, A.; García-García, R.; Leone, P.; Ballester, M.P.; Cabrera-Pastor, A.; Urios, A.; Gallego, J.-J.; Martínez-Pretel, J.-J.; Giménez-Garzó, C.; Revert, F.; et al. Selective Improvement by Rifaximin of Changes in the Immunophenotype in Patients Who Improve Minimal Hepatic Encephalopathy. J. Transl. Med. 2019, 17, 293. [Google Scholar] [CrossRef]
- Narayanan, S.; Shanker, A.; Khera, T.; Subramaniam, B. Neurofilament Light: A Narrative Review on Biomarker Utility. Fac. Rev. 2021, 10, 46. [Google Scholar] [CrossRef]
- Gaiottino, J.; Norgren, N.; Dobson, R.; Topping, J.; Nissim, A.; Malaspina, A.; Bestwick, J.P.; Monsch, A.U.; Regeniter, A.; Lindberg, R.L.; et al. Increased Neurofilament Light Chain Blood Levels in Neurodegenerative Neurological Diseases. PLoS ONE 2013, 8, e75091. [Google Scholar] [CrossRef] [PubMed]
- Osborn, K.E.; Khan, O.A.; Kresge, H.A.; Bown, C.W.; Liu, D.; Moore, E.E.; Gifford, K.A.; Acosta, L.M.Y.; Bell, S.P.; Hohman, T.J.; et al. Cerebrospinal Fluid and Plasma Neurofilament Light Relate to Abnormal Cognition. Alzheimers Dement. 2019, 11, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Giacomucci, G.; Mazzeo, S.; Bagnoli, S.; Ingannato, A.; Leccese, D.; Berti, V.; Padiglioni, S.; Galdo, G.; Ferrari, C.; Sorbi, S.; et al. Plasma Neurofilament Light Chain as a Biomarker of Alzheimer’s Disease in Subjective Cognitive Decline and Mild Cognitive Impairment. J. Neurol. 2022, 269, 4270–4280. [Google Scholar] [CrossRef] [PubMed]
- Labenz, C.; Nagel, M.; Kämper, P.; Engel, S.; Bittner, S.; Kaps, L.; Galle, P.R.; Schattenberg, J.M.; Wörns, M.-A.; Lüssi, F. Association Between Serum Levels of Neurofilament Light Chains and Minimal Hepatic Encephalopathy in Patients With Liver Cirrhosis. Clin. Transl. Gastroenterol. 2021, 12, e00419. [Google Scholar] [CrossRef]
- Bellingham, S.A.; Guo, B.B.; Coleman, B.M.; Hill, A.F. Exosomes: Vehicles for the Transfer of Toxic Proteins Associated with Neurodegenerative Diseases? Front. Physiol. 2012, 3, 124. [Google Scholar] [CrossRef]
- Gallego, J.J.; Fiorillo, A.; Casanova-Ferrer, F.; Urios, A.; Ballester, M.-P.; Durbán, L.; Megías, J.; Rubio, T.; Cabrera-Pastor, A.; Escudero-García, D.; et al. Plasma Extracellular Vesicles Play a Role in Immune System Modulation in Minimal Hepatic Encephalopathy. Int. J. Mol. Sci. 2022, 23, 12335. [Google Scholar] [CrossRef] [PubMed]
- Felipo, V.; Butterworth, R.F. Neurobiology of Ammonia. Prog. Neurobiol. 2002, 67, 259–279. [Google Scholar] [CrossRef]
- Shawcross, D.L.; Davies, N.A.; Williams, R.; Jalan, R. Systemic Inflammatory Response Exacerbates the Neuropsychological Effects of Induced Hyperammonemia in Cirrhosis. J. Hepatol. 2004, 40, 247–254. [Google Scholar] [CrossRef]
- Suhocki, P.V.; Ronald, J.S.; Diehl, A.M.E.; Murdoch, D.M.; Doraiswamy, P.M. Probing Gut-brain Links in Alzheimer’s Disease with Rifaximin. Alzheimers Dement. 2022, 8, e12225. [Google Scholar] [CrossRef]
- López-Lee, C.; Kodama, L.; Gan, L. Sex Differences in Neurodegeneration: The Role of the Immune System in Humans. Biol. Psychiatry 2022, 91, 72–80. [Google Scholar] [CrossRef]
- Aamodt, W.W.; Waligorska, T.; Shen, J.; Tropea, T.F.; Siderowf, A.; Weintraub, D.; Grossman, M.; Irwin, D.; Wolk, D.A.; Xie, S.X.; et al. Neurofilament Light Chain as a Biomarker for Cognitive Decline in Parkinson Disease. Mov. Disord. 2021, 36, 2945–2950. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Therriault, J.; Luo, J.; Ba, M.; Zhang, H.; Initiative, A.D.N. Neurofilament Light as a Biomarker of Axonal Degeneration in Patients with Mild Cognitive Impairment and Alzheimer’s Disease. J. Integr. Neurosci. 2021, 20, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gómez, M. Role of Phosphate-Activated Glutaminase in the Pathogenesis of Hepatic Encephalopathy. Metab. Brain Dis. 2005, 20, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S. The role of microbiota in hepatic encephalopathy. Gut Microbes 2014, 5, 397–403. [Google Scholar] [CrossRef] [PubMed]
- de Wit, K.; Beuers, U.; Mukha, A.; Stigter, E.C.A.; Gulersonmez, M.C.; Ramos Pittol, J.M.; Middendorp, S.; Takkenberg, R.B.; van Mil, S.W.C. Rifaximin Stimulates Nitrogen Detoxification by PXR-independent Mechanisms in Human Small Intestinal Organoids. Liver Int. 2023, 43, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, A.; Migliorati, M.; Barbanti, M.; Cipriani, S.; Palladino, G.; Distrutti, E.; Renga, B.; Fiorucci, S. Pregnane-X-Receptor Mediates the Anti-Inflammatory Activities of Rifaximin on Detoxification Pathways in Intestinal Epithelial Cells. Biochem. Pharmacol. 2010, 80, 1700–1707. [Google Scholar] [CrossRef]
- Winston, C.N.; Romero, H.K.; Ellisman, M.; Nauss, S.; Julovich, D.A.; Conger, T.; Hall, J.R.; Campana, W.; O’Bryant, S.E.; Nievergelt, C.M.; et al. Assessing Neuronal and Astrocyte Derived Exosomes From Individuals With Mild Traumatic Brain Injury for Markers of Neurodegeneration and Cytotoxic Activity. Front. Neurosci. 2019, 13, 1005. [Google Scholar] [CrossRef]
- Pulliam, L.; Sun, B.; Mustapic, M.; Chawla, S.; Kapogiannis, D. Plasma Neuronal Exosomes Serve as Biomarkers of Cognitive Impairment in HIV Infection and Alzheimer’s Disease. J. Neurovirol. 2019, 25, 702–709. [Google Scholar] [CrossRef]
- Lachenal, G.; Pernet-Gallay, K.; Chivet, M.; Hemming, F.J.; Belly, A.; Bodon, G.; Blot, B.; Haase, G.; Goldberg, Y.; Sadoul, R. Release of Exosomes from Differentiated Neurons and Its Regulation by Synaptic Glutamatergic Activity. Mol. Cell. Neurosci. 2011, 46, 409–418. [Google Scholar] [CrossRef]
- ElMlili, N.; Boix, J.; Ahabrach, H.; Rodrigo, R.; Errami, M.; Felipo, V. Chronic Hyperammonemia Induces Tonic Activation of NMDA Receptors in Cerebellum. J. Neurochem. 2010, 112, 1005–1014. [Google Scholar] [CrossRef]
- Felipo, V.; Hermenegildo, C.; Montoliu, C.; Llansola, M.; Miñana, M.D. Neurotoxicity of Ammonia and Glutamate: Molecular Mechanisms and Prevention. Neurotoxicology 1998, 19, 675–681. [Google Scholar]
- Leone, P.; Mincheva, G.; Balzano, T.; Malaguarnera, M.; Felipo, V.; Llansola, M. Rifaximin Improves Spatial Learning and Memory Impairment in Rats with Liver Damage-Associated Neuroinflammation. Biomedicines 2022, 10, 1263. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Barrett, A.C.; Bortey, E.; Paterson, C.; Forbes, W.P. Prolonged Remission from Hepatic Encephalopathy with Rifaximin: Results of a Placebo Crossover Analysis. Aliment. Pharmacol. Ther. 2015, 41, 39–45. [Google Scholar] [CrossRef]
- Mullen, K.D.; Sanyal, A.J.; Bass, N.M.; Poordad, F.F.; Sheikh, M.Y.; Frederick, R.T.; Bortey, E.; Forbes, W.P. Rifaximin Is Safe and Well Tolerated for Long-Term Maintenance of Remission From Overt Hepatic Encephalopathy. Clin. Gastroenterol. Hepatol. 2014, 12, 1390–1397.e2. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Heuman, D.M.; Wade, J.B.; Gibson, D.P.; Saeian, K.; Wegelin, J.A.; Hafeezullah, M.; Bell, D.E.; Sterling, R.K.; Stravitz, R.T.; et al. Rifaximin Improves Driving Simulator Performance in a Randomized Trial of Patients With Minimal Hepatic Encephalopathy. Gastroenterology 2011, 140, 478–487.e1. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Heuman, D.M.; Sanyal, A.J.; Hylemon, P.B.; Sterling, R.K.; Stravitz, R.T.; Fuchs, M.; Ridlon, J.M.; Daita, K.; Monteith, P.; et al. Modulation of the Metabiome by Rifaximin in Patients with Cirrhosis and Minimal Hepatic Encephalopathy. PLoS ONE 2013, 8, e60042. [Google Scholar] [CrossRef] [PubMed]
- Ballester, M.-P.; Gallego, J.-J.; Fiorillo, A.; Casanova-Ferrer, F.; Giménez-Garzó, C.; Escudero-García, D.; Tosca, J.; Ríos, M.-P.; Montón, C.; Durbán, L.; et al. Metabolic Syndrome Is Associated with Poor Response to Rifaximin in Minimal Hepatic Encephalopathy. Sci. Rep. 2022, 12, 2463. [Google Scholar] [CrossRef]



| Variables | Control (n = 26) | Cirrhotic Patients | MHE Patients Treated with Rifaximin (Before Treatment) | ||
|---|---|---|---|---|---|
| NMHE (n = 31) | MHE (n = 40) | R0 (n = 16) | NR0 (n = 15) | ||
| Sex, n (%) | |||||
| Male | 14 (54) | 27 (87) | 31 (78) | 13 (81) | 12 (80) |
| Female | 12 (46) | 4 (13) | 9 (22) | 3 (19) | 3 (20) |
| Age (years) a | 61.7 ± 1.1 | 60.3 ± 1.4 | 63.5 ± 1.2 | 59.3 ± 1.6 | 67.1 ± 1.7 αα/ββ |
| Etiology of cirrhosis, n (%) | |||||
| Alcohol | - | 19 (61) | 20 (50) | 11 (69) | 6 (40) |
| HBV/HCV | - | 7 (23) | 9 (22) | 3 (19) | 4 (27) |
| NASH | - | 2 (6) | 8 (20) | 1 (6) | 5 (33) |
| Others | - | 3 (10) | 3 (8) | 1 (6) | 0 (0) |
| Child–Pugh score (A/B/C) | - | 24/5/2 | 25/13/2 | 10/6/0 | 9/5/1 |
| MELD score a | - | 8.8 ± 0.5 | 9.9 ± 0.6 | 9.5 ± 0.5 | 9.9 ± 1.3 |
| Clinical parameters | |||||
| Ammonia (µM) a | 10.7 ± 1.0 | 23.0 ± 4.5 * | 40.2 ± 5.2 ***/α | 45.6 ± 10.5 | 32.5 ± 6.8 |
| AST (U/L) a | 23.8 ± 1.4 | 36.1 ± 3.3 ** | 45.3 ± 5.6 ** | 42.9 ± 5.7 * | 35 ± 5.1 |
| ALT (U/L) a | 24.2 ± 1.5 | 29.1 ± 1.9 | 34.1 ± 3.9 | 33.6 ± 4.3 | 27.3 ± 2.2 |
| GGT (U/L) a | 30.4 ± 5.2 | 71.6 ± 14.1 * | 79.6 ± 9.8 *** | 83.9 ± 13.9 ** | 73.5 ± 16.3 |
| Albumin (g/dL) a | 4.4 ± 0.1 | 4.0 ± 0.1 | 3.7 ± 0.1 *** | 3.7 ± 0.1 *** | 3.9 ± 0.1 ** |
| Bilirubin (mg/dL) a | 0.58 ± 0.06 | 1.26 ± 0.19 | 1.64 ± 0.3 * | 1.44 ± 0.25 * | 1.85 ± 0.74 |
| Creatinine (mg/dL) a | 0.84 ± 0.04 | 0.87 ± 0.07 | 0.85 ± 0.04 | 0.79 ± 0.05 | 0.94 ± 0.06 |
| Platelets (·109/L) a | 237.1 ± 15.5 | 135.1 ± 12.8 *** | 125.1 ± 9.9 *** | 105.3 ± 10.3 *** | 131.1 ± 15.1 *** |
| NfL Levels | Control | Cirrhotic Patients | MHE Patients Treated with Rifaximin | ||||
|---|---|---|---|---|---|---|---|
| NMHE | MHE | R0 | R6 | NR0 | NR6 | ||
| Plasma a | 11.6 ± 0.5 | 12.1 ± 0.8 | 17.8 ± 1.9 **/α | 16.1 ± 2.7 | 11.9 ± 1.8 δ | 19.6 ± 2.8 */α | 23.9 ± 4.3 */α/β |
| EVs b | 100.2 ± 10.3 | 117.2 ± 4.7 | 118.0 ± 8.6 | 102.1 ± 9.4 | 76.2 ± 6.43 ααα/δ | 131.9 ± 13.12 | 86.8 ± 10.5 α/δδδ |
| Correlations with Plasma NfL Levels | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Cirrhotic Patients | NMHE Patients | MHE Patients | R0 Patients | NR0 Patients | |||||
| Correlation Coefficient | p Value | Correlation Coefficient | p Value | Correlation Coefficient | p Value | Correlation Coefficient | p Value | Correlation Coefficient | pValue | |
| PHES | −0.458 | <0.0001 | −0.510 | 0.001 | −0.527 | 0.010 | −0.642 | 0.010 | ||
| Stroop Test | ||||||||||
| Congruent | −0.314 | 0.010 | ||||||||
| Neutral | −0.356 | 0.003 | ||||||||
| Incongruent | −0.305 | 0.012 | ||||||||
| d2 Test | ||||||||||
| TR values | −0.467 | <0.0001 | −0.521 | 0.004 | −0.720 | 0.013 | ||||
| TA values | −0.487 | <0.0001 | −0.567 | 0.001 | −0.677 | 0.011 | −0.656 | 0.029 | ||
| TOT values | −0.524 | <0.0001 | −0.605 | 0.001 | −0.648 | 0.017 | −0.710 | 0.014 | ||
| CON values | −0.503 | <0.0001 | −0.608 | <0.0001 | −0.763 | 0.002 | −0.663 | 0.026 | ||
| Coordination tests | ||||||||||
| Bimanual | 0.468 | <0.0001 | 0.406 | 0.013 | 0.517 | 0.049 | ||||
| Visuomotor | 0.557 | <0.0001 | 0.513 | 0.001 | 0.848 | <0.0001 | ||||
| Oral SDMT test | ||||||||||
| Scaled score | −0.293 | 0.014 | ||||||||
| Biochemical parameters | ||||||||||
| Ammonia | 0.320 | 0.008 | 0.363 | 0.030 | 0.529 | 0.042 | ||||
| IL-13 | −0.468 | 0.014 | −0.825 | 0.006 | ||||||
| CCL20 | 0.438 | 0.001 | 0.402 | 0.020 | 0.829 | <0.0001 | ||||
| IL-22 | 0.259 | 0.046 | 0.695 | 0.026 | ||||||
| CX3CL1 | 0.445 | <0.0001 | 0.355 | 0.042 | ||||||
| NfL (EVs) | 0.519 | <0.0001 | 0.589 | <0.0001 | ||||||
| Correlations with NfL levels in EVs | ||||||||||
| d2 Test | ||||||||||
| TR values | −0.385 | 0.011 | −0.447 | 0.010 | −0.733 | 0.007 | ||||
| TA values | −0.711 | 0.010 | ||||||||
| TOT values | −0.309 | 0.044 | −0.659 | 0.020 | ||||||
| Biochemical parameters | ||||||||||
| IL-22 | 0.404 | 0.006 | −0.569 | 0.042 | 0.486 | 0.005 | 0.539 | 0.047 | ||
| CX3CL1 | −0.392 | 0.015 | −0.415 | 0.028 | ||||||
| NfL (plasma) | 0.519 | 0.000 | 0.589 | <0.0001 | 0.562 | 0.023 | ||||
| Correlation a with PHES Score | Receiver Operating Characteristic (ROC) Curves b | ||||||
|---|---|---|---|---|---|---|---|
| Parameters | Correlation Coefficient | p Value | AUROC (95% CI) | p Value | Cutoff | Sensitivity | Specificity |
| IL-6 | 0.013 | 0.945 | 0.463 (0.256–0.670) | 0.751 | |||
| IL-18 | −0.045 | 0.740 | 0.444 (0.294–0.594) | 0.479 | |||
| IL-13 | 0.032 | 0.872 | 0.602 (0.332–0.873) | 0.389 | |||
| CCL20 | −0.136 | 0.332 | 0.558 (0.403–0.712) | 0.486 | |||
| IL-22 | −0.165 | 0.213 | 0.644 (0.500–0.788) | 0.060 | |||
| TGF-β | −0.025 | 0.856 | 0.548 (0.394–0.701) | 0.546 | |||
| CCL2 | −0.176 | 0.190 | 0.640 (0.495–0.784) | 0.074 | |||
| Ammonia | −0.270 | 0.025 | 0.670 (0.539–0.801) | 0.016 | 18.2 c | 70 | 69 |
| CX3CL1 | −0.545 | <0.0001 | 0.860 (0.769–0.951) | 0.000 | 504 d | 74 | 89 |
| NfL | −0.331 | 0.005 | 0.666 (0.539–0.792) | 0.019 | 12.6 d | 61 | 68 |
| Univariate Logistic Regression Analyses | ||
|---|---|---|
| Independent Variables | OR (95% CI) | p Value |
| AST | 1.015 (0.991–1.039) | 0.229 |
| ALT | 1.016 (0.984–1.048) | 0.329 |
| GGT | 1.002 (0.993–1.011) | 0.637 |
| Albumin | 0.430 (0.168–1.098) | 0.078 |
| Bilirubin | 1.225 (0.806–1.861) | 0.342 |
| Creatinine | 0.787 (0.168–3.691) | 0.761 |
| Platelets | 0.998 (0.991–1.005) | 0.525 |
| MELD | 1.124 (0.948–1.333) | 0.179 |
| Ammonia | 1.023 (1.002–1.045) | 0.029 |
| NfL | 1.115 (1.022–1.217) | 0.014 |
| Multivariate Logistic Regression Analysis | ||
| Independent Variables | OR (95% CI) | p Value |
| NfL | 1.105 (1.007–1.213) | 0.034 |
| Ammonia | 1.013 (0.991–1.035) | 0.244 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorillo, A.; Gallego, J.J.; Casanova-Ferrer, F.; Urios, A.; Ballester, M.-P.; San Miguel, T.; Megías, J.; Kosenko, E.; Tosca, J.; Rios, M.-P.; et al. Neurofilament Light Chain Protein in Plasma and Extracellular Vesicles Is Associated with Minimal Hepatic Encephalopathy and Responses to Rifaximin Treatment in Cirrhotic Patients. Int. J. Mol. Sci. 2023, 24, 14727. https://doi.org/10.3390/ijms241914727
Fiorillo A, Gallego JJ, Casanova-Ferrer F, Urios A, Ballester M-P, San Miguel T, Megías J, Kosenko E, Tosca J, Rios M-P, et al. Neurofilament Light Chain Protein in Plasma and Extracellular Vesicles Is Associated with Minimal Hepatic Encephalopathy and Responses to Rifaximin Treatment in Cirrhotic Patients. International Journal of Molecular Sciences. 2023; 24(19):14727. https://doi.org/10.3390/ijms241914727
Chicago/Turabian StyleFiorillo, Alessandra, Juan José Gallego, Franc Casanova-Ferrer, Amparo Urios, María-Pilar Ballester, Teresa San Miguel, Javier Megías, Elena Kosenko, Joan Tosca, Maria-Pilar Rios, and et al. 2023. "Neurofilament Light Chain Protein in Plasma and Extracellular Vesicles Is Associated with Minimal Hepatic Encephalopathy and Responses to Rifaximin Treatment in Cirrhotic Patients" International Journal of Molecular Sciences 24, no. 19: 14727. https://doi.org/10.3390/ijms241914727
APA StyleFiorillo, A., Gallego, J. J., Casanova-Ferrer, F., Urios, A., Ballester, M. -P., San Miguel, T., Megías, J., Kosenko, E., Tosca, J., Rios, M. -P., Escudero-García, D., & Montoliu, C. (2023). Neurofilament Light Chain Protein in Plasma and Extracellular Vesicles Is Associated with Minimal Hepatic Encephalopathy and Responses to Rifaximin Treatment in Cirrhotic Patients. International Journal of Molecular Sciences, 24(19), 14727. https://doi.org/10.3390/ijms241914727