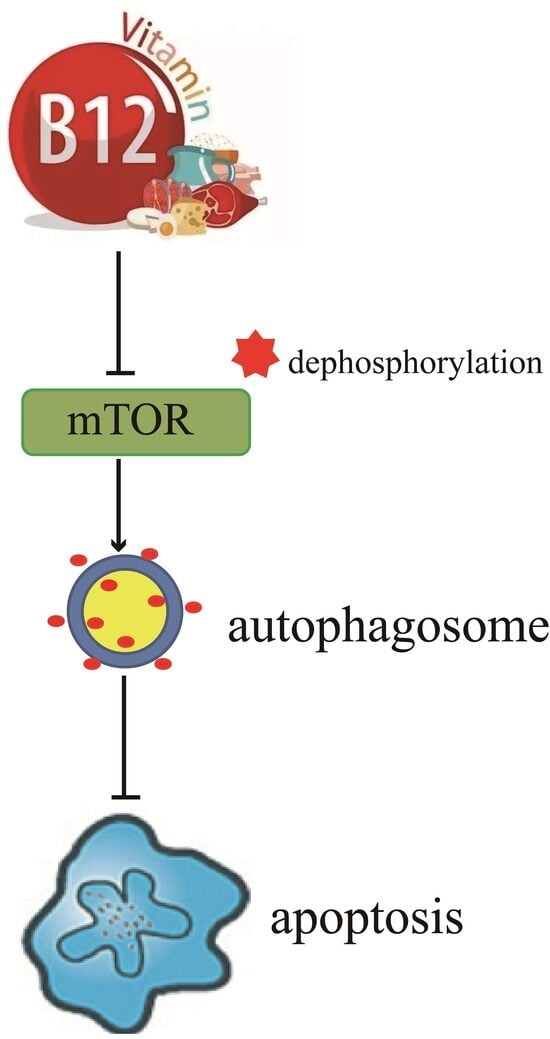
Vitamin B12-Induced Autophagy Alleviates High Glucose-Mediated Apoptosis of Islet β Cells
Abstract
:1. Introduction
2. Results
2.1. Vitamin B12 Inhibits High Glucose-Induced Apoptosis
2.2. Vitamin B12 Induces Autophagy under Normal Culture Conditions
2.3. Vitamin B12 Induces Autophagy under High Glucose Stress
2.4. 3-Methyladenine Suppresses Vitamin B12-Induced Autophagy under High Glucose Stress
2.5. 3-MA Inhibits Vitamin B12-Induced Cytoprotective Autophagy against Apoptosis
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture
4.3. Immunofluorescence Staining
4.4. Western Blotting
4.5. Cell Apoptosis Assay
4.6. Cell Viability Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obeid, R.; Fedosov, S.N.; Nexo, E. Cobalamin coenzyme forms are not likely to be superior to cyano- and hydroxyl-cobalamin in prevention or treatment of cobalamin deficiency. Mol. Nutr. Food Res. 2015, 59, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Lenhert, P.G.; Hodgkin, D.C. Structure of the 5,6-dimethyl-benzimidazolylcobamide coenzyme. Nature 1961, 192, 937–938. [Google Scholar] [CrossRef] [PubMed]
- Infante, M.; Leoni, M.; Caprio, M.; Fabbri, A. Long-term metformin therapy and vitamin B12 deficiency: An association to bear in mind. World J. Diabetes 2021, 12, 916–931. [Google Scholar] [CrossRef] [PubMed]
- Pieczyńska, J.; Płaczkowska, S.; Sozański, R.; Skórska, K.; Sołtysik, M. Effect of nickel on red blood cell parameters and on serum vitamin B12, folate and homocysteine concentrations during pregnancy with and without anemia. J. Trace Elem. Med. Biol. 2021, 68, 126839. [Google Scholar] [CrossRef]
- Elbarbary, N.S.; Ismail, E.A.R.; Zaki, M.A.; Darwish, Y.W.; Ibrahim, M.Z.; El-Hamamsy, M. Vitamin B complex supplementation as a homocysteine-lowering therapy for early stage diabetic nephropathy in pediatric patients with type 1 diabetes: A randomized controlled trial. Clin. Nutr. 2020, 39, 49–56. [Google Scholar] [CrossRef]
- Yang, P.; Feng, J.; Peng, Q.; Liu, X.; Fan, Z. Advanced Glycation End Products: Potential Mechanism and Therapeutic Target in Cardiovascular Complications under Diabetes. Oxid. Med. Cell. Longev. 2019, 2019, 9570616. [Google Scholar] [CrossRef]
- Wu, S.; Feng, P.; Li, W.; Zhuo, S.; Lu, W.; Chen, P.; Sui, Y.; Fang, S.; Yang, Z.; Ye, Y. Dietary Folate, Vitamin B6, and Vitamin B12 and Risk of Cardiovascular Diseases among Individuals with Type 2 Diabetes: A Case-Control Study. Ann. Nutr. Metab. 2023, 79, 5–15. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Nolan, C.J.; Damm, P.; Prentki, M. Type 2 diabetes across generations: From pathophysiology to prevention and management. Lancet 2011, 378, 169–181. [Google Scholar] [CrossRef]
- Joaquim, L.; Faria, A.; Loureiro, H.; Matafome, P. Benefits, mechanisms, and risks of intermittent fasting in metabolic syndrome and type 2 diabetes. J. Physiol. Biochem. 2022, 78, 295–305. [Google Scholar] [CrossRef]
- Tao, Z.; Shi, A.; Zhao, J. Epidemiological Perspectives of Diabetes. Cell Biochem. Biophys. 2015, 73, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, J.K.; Moshfegh, A.J.; Holden, J.M.; Harris, E. USDA food and nutrient databases provide the infrastructure for food and nutrition research, policy, and practice. J. Nutr. 2013, 143, 241S–249S. [Google Scholar] [CrossRef]
- American Diabetes Association. 2 Classification and diagnosis of diabetes. Diabetes Care 2015, 38, S8–S16. [Google Scholar] [CrossRef] [PubMed]
- Min, J.Y.; Min, K.B. The Folate-Vitamin B12 Interaction, Low Hemoglobin, and the Mortality Risk from Alzheimer’s Disease. J. Alzheimers Dis. 2016, 52, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Mursleen, M.T.; Riaz, S. Implication of homocysteine in diabetes and impact of folate and vitamin B12 in diabetic population. Diabetes Metab. Syndr. 2017, 11 (Suppl. S1), S141–S146. [Google Scholar] [CrossRef]
- Prentice, R.L.; Mossavar-Rahmani, Y.; Huang, Y.; Van Horn, L.; Beresford, S.A.; Caan, B.; Tinker, L.; Schoeller, D.; Bingham, S.; Eaton, C.B.; et al. Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment by using recovery biomarkers. Am. J. Epidemiol. 2011, 174, 591–603. [Google Scholar] [CrossRef]
- Salimi, L.; Akbari, A.; Jabbari, N.; Mojarad, B.; Vahhabi, A.; Szafert, S.; Kalashani, S.A.; Soraya, H.; Nawaz, M.; Rezaie, J. Synergies in exosomes and autophagy pathways for cellular homeostasis and metastasis of tumor cells. Cell Biosci. 2020, 10, 64. [Google Scholar] [CrossRef]
- López-Otín, C.; Kroemer, G. Hallmarks of Health. Cell 2021, 184, 33–63. [Google Scholar] [CrossRef]
- Yang, S.C.; Hsu, C.Y.; Chou, W.L.; Fang, J.Y.; Chuang, S.Y. Bioactive Agent Discovery from the Natural Compounds for the Treatment of Type 2 Diabetes Rat Model. Molecules 2020, 25, 5713. [Google Scholar] [CrossRef]
- Taucher, E.; Mykoliuk, I.; Fediuk, M.; Smolle-Juettner, F.M. Autophagy, Oxidative Stress and Cancer Development. Cancers 2022, 14, 1637. [Google Scholar] [CrossRef]
- Semwal, D.K.; Kumar, A.; Aswal, S.; Chauhan, A.; Semwal, R.B. Protective and therapeutic effects of natural products against diabetes mellitus via regenerating pancreatic β-cells and restoring their dysfunction. Phytother. Res. 2021, 35, 1218–1229. [Google Scholar] [CrossRef]
- Zhang, X.; Su, Q.; Zhou, J.; Yang, Z.; Liu, Z.; Ji, L.; Gao, H.; Jiang, G. To betray or to fight? The dual identity of the mitochondria in cancer. Future Oncol. 2021, 17, 723–743. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B. Autophagy in mammalian development and differentiation. Nat. Cell Biol. 2010, 12, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Doets, E.L.; In’t Veld, P.H.; Szczecińska, A.; Dhonukshe-Rutten, R.A.; Cavelaars, A.E.; van’t Veer, P.; Brzozowska, A.; de Groot, L.C. Systematic review on daily vitamin B12 losses and bioavailability for deriving recommendations on vitamin B12 intake with the factorial approach. Ann. Nutr. Metab. 2013, 62, 311–322. [Google Scholar] [CrossRef]
- Lu, Q.; Yan, S.; Sun, H.; Wang, W.; Li, Y.; Yang, X.; Jiang, X.; Che, Y.; Xi, Z. Akt inhibition attenuates rasfonin-induced autophagy and apoptosis through the glycolytic pathway in renal cancer cells. Cell Death Dis. 2015, 6, e2005. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Wolgamott, L.; Yu, Y.; Blenis, J.; Yoon, S.O. Glycogen synthase kinase (GSK)-3 promotes p70 ribosomal protein S6 kinase (p70S6K) activity and cell proliferation. Proc. Natl. Acad. Sci. USA 2011, 108, E1204–E1213. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Derrick, S.A.; Kristo, A.S.; Reaves, S.K.; Sikalidis, A.K. Effects of Dietary Red Raspberry Consumption on Pre-Diabetes and Type 2 Diabetes Mellitus Parameters. Int. J. Environ. Res. Public Health 2021, 18, 9364. [Google Scholar] [CrossRef]
- Ilonen, J.; Lempainen, J.; Veijola, R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 635–650. [Google Scholar] [CrossRef]
- Magkos, F.; Yannakoulia, M.; Chan, J.L.; Mantzoros, C.S. Management of the metabolic syndrome and type 2 diabetes through lifestyle modification. Annu. Rev. Nutr. 2009, 29, 223–256. [Google Scholar] [CrossRef]
- Tay, J.; Thompson, C.H.; Brinkworth, G.D. Glycemic Variability: Assessing Glycemia Differently and the Implications for Dietary Management of Diabetes. Annu. Rev. Nutr. 2015, 35, 389–424. [Google Scholar] [CrossRef] [PubMed]
- Antosik, K.; Borowiec, M. Genetic Factors of Diabetes. Arch. Immunol. Ther. Exp. 2016, 64, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.J.; Wray, L.A. Physical disability trajectories in older Americans with and without diabetes: The role of age, gender, race or ethnicity, and education. Gerontologist 2011, 51, 51–63. [Google Scholar] [CrossRef]
- Ortega, M.A.; Pekarek, L.; Garcia-Montero, C.; Fraile-Martinez, O.; Saez, M.A.; Asúnsolo, A.; Alvarez-Mon, M.A.; Monserrat, J.; Coca, S.; Toledo-Lobo, M.V.; et al. Prognostic role of IRS-4 in the survival of patients with pancreatic cancer. Histol. Histopathol. 2022, 37, 449–459. [Google Scholar]
- Jousilahti, P.; Vartiainen, E.; Tuomilehto, J.; Puska, P. Sex, age, cardiovascular risk factors, and coronary heart disease: A prospective follow-up study of 14786 middle-aged men and women in Finland. Circulation 1999, 99, 1165–1172. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing a Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef]
- Palacios, O.M.; Kramer, M.; Maki, K.C. Diet and prevention of type 2 diabetes mellitus: Beyond weight loss and exercise. Expert Rev. Endocrinol. Metab. 2019, 14, 1–12. [Google Scholar] [CrossRef]
- Obeid, R.; Heil, S.G.; Verhoeven, M.M.A.; van den Heuvel, E.G.H.M.; de Groot, L.C.P.G.M.; Eussen, S.J.P.M. Vitamin B12 Intake From Animal Foods, Biomarkers, and Health Aspects. Front. Nutr. 2019, 6, 93. [Google Scholar] [CrossRef]
- Xu, F.; Hua, C.; Tautenhahn, H.M.; Dirsch, O.; Dahmen, U. The Role of Autophagy for the Regeneration of the Aging Liver. Int. J. Mol. Sci. 2020, 21, 3606. [Google Scholar] [CrossRef] [PubMed]
- Licheva, M.; Raman, B.; Kraft, C.; Reggiori, F. Phosphoregulation of the autophagy machinery by kinases and phosphatases. Autophagy 2022, 18, 104–123. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Han, X.; Han, X. The cardioprotection of the insulin-mediated PI3K/Akt/mTOR signaling pathway. Am. J. Cardiovasc. Drugs. 2014, 14, 433–442. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, J.; Park, K.; Lee, M.S. β-cell autophagy: Mechanism and role in β-cell dysfunction. Mol. Metab. 2019, 27S, S92–S103. [Google Scholar] [CrossRef]
- Elizabeth, M.M.; Alarcon-Aguilar, J.F.; Clara, O.C.; Escobar-Villanueva, D.C.M. Pancreatic β-Cells and Type 2 Diabetes Development. Curr. Diabetes Rev. 2017, 13, 108–121. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chuang, L.M.; Lin, J.W.; Chen, S.T.; Lai, M.S.; Chang, C.H. Cardiovascular risks associated with second-line oral antidiabetic agents added to metformin in patients with Type 2 diabetes: A nationwide cohort study. Diabet. Med. 2015, 32, 1460–1469. [Google Scholar] [CrossRef]
- Solovieva, M.; Shatalin, Y.; Fadeev, R.; Krestinina, O.; Baburina, Y.; Kruglov, A.; Kharechkina, E.; Kobyakova, M.; Rogachevsky, V.; Shishkova, E.; et al. Vitamin B12b Enhances the Cytotoxicity of Diethyldithiocarbamate in a Synergistic Manner, Inducing the Paraptosis-Like Death of Human Larynx Carcinoma Cells. Biomolecules 2020, 10, 69. [Google Scholar] [CrossRef]
- Aroda, V.R.; Edelstein, S.L.; Goldberg, R.B.; Knowler, W.C.; Marcovina, S.M.; Orchard, T.J.; Bray, G.A.; Schade, D.S.; Temprosa, M.G.; White, N.H.; et al. Long-term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. J. Clin. Endocrinol. Metab. 2016, 101, 1754–1761. [Google Scholar] [CrossRef]
- Klimontov, V.V.; Saik, O.V.; Korbut, A.I. Glucose Variability: How Does It Work? Int. J. Mol. Sci. 2021, 22, 7783. [Google Scholar] [CrossRef]
- Ohnishi, M.; Hasegawa, G.; Yamasaki, M.; Obayashi, H.; Fukui, M.; Nakajima, T.; Ichida, Y.; Ohse, H.; Mogami, S.; Yoshikawa, T.; et al. Integrin-linked kinase acts as a pro-survival factor against high glucose-associated osmotic stress in human mesangial cells. Nephrol. Dial. Transplant. 2006, 21, 1786–1793. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chu, L.; Zhou, X.; Xu, T.; Shen, Q.; Li, T.; Wu, Y. Vitamin B12-Induced Autophagy Alleviates High Glucose-Mediated Apoptosis of Islet β Cells. Int. J. Mol. Sci. 2023, 24, 15217. https://doi.org/10.3390/ijms242015217
Zhang Y, Chu L, Zhou X, Xu T, Shen Q, Li T, Wu Y. Vitamin B12-Induced Autophagy Alleviates High Glucose-Mediated Apoptosis of Islet β Cells. International Journal of Molecular Sciences. 2023; 24(20):15217. https://doi.org/10.3390/ijms242015217
Chicago/Turabian StyleZhang, Yu, Ling Chu, Xi’an Zhou, Tingxia Xu, Qingwu Shen, Tao Li, and Yanyang Wu. 2023. "Vitamin B12-Induced Autophagy Alleviates High Glucose-Mediated Apoptosis of Islet β Cells" International Journal of Molecular Sciences 24, no. 20: 15217. https://doi.org/10.3390/ijms242015217
APA StyleZhang, Y., Chu, L., Zhou, X., Xu, T., Shen, Q., Li, T., & Wu, Y. (2023). Vitamin B12-Induced Autophagy Alleviates High Glucose-Mediated Apoptosis of Islet β Cells. International Journal of Molecular Sciences, 24(20), 15217. https://doi.org/10.3390/ijms242015217