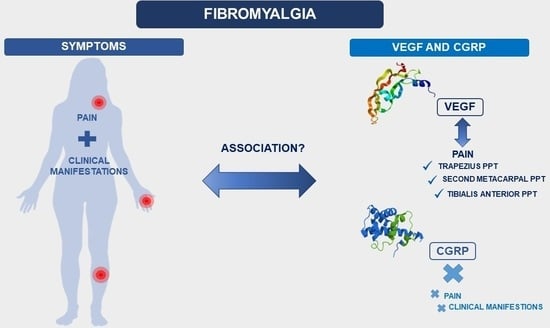
Serum VEGF and CGRP Biomarkers: Relationships with Pain Intensity, Electric Pain, Pressure Pain Threshold, and Clinical Symptoms in Fibromyalgia—An Observational Study
Abstract
:1. Introduction
2. Results
2.1. Study Variables in Women with FM
2.2. Associations of Serum VEGF Levels with FIQ-R, CSI, MFI, BAI, VAS, Electric Pain Threshold, Electric Pain Magnitude, and PPTs in Women with FM
2.3. Associations of Serum CGRP Levels with FIQ-R, CSI, MFI, BAI, VAS, Electric Pain Threshold, Electric Pain Magnitude, and PPTs in Women with FM
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Outcome Measures
4.2.1. Questionnaires
4.2.2. Pain Assessment
4.2.3. Blood Collection and Measurement of Serum VEGF and CGRP Levels
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maffei, M.E. Fibromyalgia: Recent advances in diagnosis, classification, pharmacotherapy and alternative remedies. Int. J. Mol. Sci. 2020, 21, 7877. [Google Scholar] [CrossRef] [PubMed]
- D’Agnelli, S.; Arendt-Nielsen, L.; Gerra, M.C.; Zatorri, K.; Boggiani, L.; Baciarello, M.; Bignami, E. Fibromyalgia: Genetics and epigenetics insights may provide the basis for the development of diagnostic biomarkers. Mol. Pain. 2019, 15, 1744806918819944. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.P.; Santo, A.d.S.d.E.; Berssaneti, A.A.; Matsutani, L.A.; Yuan, S.L.K. Prevalence of fibromyalgia: Literature review update. Rev. Bras. Reumatol. 2017, 57, 356–363. [Google Scholar] [CrossRef]
- Cabo-Meseguer, A.; Cerdá-Olmedo, G.; Trillo-Mata, J.L. Fibromyalgia: Prevalence, epidemiologic profiles and economic costs. Med. Clin. 2017, 49, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, V.; Sirotti, S.; Romano, M.E.; Marotto, D.; Ablin, J.N.; Salaffi, F.; Sarzi-Puttini, P. Fibromyalgia: One year in review 2022. Clin. Exp. Rheumatol. 2022, 8, 1065–1072. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Giorgi, V.; Marotto, D.; Atzeni, F. Fibromyalgia: An update on clinical characteristics, aetiopathogenesis and treatment. Nat. Rev. Rheumatol. 2020, 16, 645–660. [Google Scholar] [CrossRef]
- Bair, M.J.; Krebs, E.E. In the clinic®: Fibromyalgia. Ann. Intern. Med. 2020, 172, ITC33–ITC48. [Google Scholar] [CrossRef]
- Queiroz, L.P. Worldwide epidemiology of fibromyalgia. Curr. Pain Headache Rep. 2013, 17, 356. [Google Scholar] [CrossRef]
- Arslan, D. Interactions Between the Painful Disorders and the Autonomic Nervous System. Ağrı. J. Turk. Soc. Algol. 2022, 34, 155–165. [Google Scholar] [CrossRef]
- Dos Santos, J.M.; Rodrigues Lacerda, A.C.; Ribeiro, V.G.C.; Scheidt Figueiredo, P.H.; Fonseca, S.F.; da Silva Lage, V.K.; Costa, H.S.; Pereira Lima, V.; Sañudo, B.; Bernardo-Filho, M.; et al. Oxidative Stress Biomarkers and Quality of Life Are Contributing Factors of Muscle Pain and Lean Body Mass in Patients with Fibromyalgia. Biology 2022, 11, 935. [Google Scholar] [CrossRef]
- García Rodríguez, D.F.; Abud Mendoza, C. Physiopathology of fibromyalgia. Reumatol. Clin. 2020, 16, 191–194. [Google Scholar] [CrossRef] [PubMed]
- On, A.Y.; Tanigor, G.; Baydar, D.A. Relationships of autonomic dysfunction with disease severity and neuropathic pain features in fibromyalgia: Is it really a sympathetically maintained neuropathic pain? Korean J. Pain. 2022, 35, 327–335. [Google Scholar] [CrossRef]
- Choi, D.H.; Kim, H.S. Quantitative analysis of nailfold capillary morphology in patients with fibromyalgia. Korean J. Intern. Med. 2015, 30, 531–537. [Google Scholar] [CrossRef]
- Albrecht, P.J.; Hou, Q.; Argoff, C.E.; Storey, J.R.; Wymer, J.P.; Rice, F.L. Excessive peptidergic sensory innervation of cutaneous arteriole-venule shunts (AVS) in the palmar glabrous skin of fibromyalgia patients: Implications for widespread deep tissue pain and fatigue. Pain Med. 2013, 14, 895–915. [Google Scholar] [CrossRef] [PubMed]
- Chinn, S.; Caldwell, W.; Gritsenko, K. Fibromyalgia Pathogenesis and Treatment Options Update. Curr. Pain Headache Rep. 2016, 20, 25. [Google Scholar] [CrossRef]
- Nijs, J.; Paul van Wilgen, C.; Van Oosterwijck, J.; Van Ittersum, M.; Meeus, M. How to explain central sensitization to patients with “unexplained” chronic musculoskeletal pain: Practice guidelines. Man. Ther. 2011, 16, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Cagnie, B.; Coppieters, I.; Denecker, S.; Six, J.; Danneels, L.; Meeus, M. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin. Arthritis Rheum. 2014, 44, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Favretti, M.; Iannuccelli, C.; Di Franco, M. Pain Biomarkers in Fibromyalgia Syndrome: Current Understanding and Future Directions. Int. J. Mol. Sci. 2023, 24, 10443. [Google Scholar] [CrossRef] [PubMed]
- Meeus, M.; Goubert, D.; De Backer, F.; Struyf, F.; Hermans, L.; Coppieters, I.; De Wandele, I.; Da Silva, H.; Calders, P. Heart rate variability in patients with fibromyalgia and patients with chronic fatigue syndrome: A systematic review. Semin. Arthritis Rheum. 2013, 43, 279–287. [Google Scholar] [CrossRef]
- Hulse, R.P. Role of VEGF-A in chronic pain. Oncotarget 2017, 8, 10775–10776. [Google Scholar] [CrossRef]
- Fila, M.; Sobczuk, A.; Pawlowska, E.; Blasiak, J. Epigenetic Connection of the Calcitonin Gene-Related Peptide and Its Potential in Migraine. Int. J. Mol. Sci. 2022, 23, 6151. [Google Scholar] [CrossRef]
- Iyengar, S.; Ossipov, M.H.; Johnson, K.W. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain 2017, 158, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Korucu, R.U.; Karadağ, A.; Taş, A.; Özmen, E.; Hayta, E.; Siliğ, Y. Serum calcitonin gene-related peptide and receptor protein levels in patients with fibromyalgia syndrome: A cross-sectional study. Arch. Rheumatol. 2020, 35, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Blanco, I.; Janciauskiene, S.; Nita, I.; Fernández-Bustillo, E.; Cárcaba, V.; Gallo, C.; Alvarez-Rico, M.; de Serres, F.; Beridze, N. Low plasma levels of monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-alpha (TNFα), and vascular endothelial growth factor (VEGF) in patients with alpha1-antitrypsin deficiency-related fibromyalgia. Clin. Rheumatol. 2010, 29, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Kim, K.S.; Lee, Y.S.; Park, S.H.; Choe, J.Y. Arterial stiffness and proinflammatory cytokines in fibromyalgia syndrome. Clin. Exp. Rheumatol. 2010, 28, S71–S77. [Google Scholar] [PubMed]
- Karadağ, A.; Hayta, E.; Çelik, V.K.; Bakir, S. Serum vascular endothelial growth factor and vascular endothelial growth factor receptor-1 levels in patients with fibromyalgia syndrome. Arch. Rheumatol. 2019, 34, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Jeschonneck, M.; Grohmann, G.; Hein, G.; Sprott, H. Abnormal microcirculation and temperature in skin above tender points in patients with fibromyalgia. Rheumatology 2000, 39, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Morf, S.; Amann-Vesti, B.; Forster, A.; Franzeck, U.K.; Koppensteiner, R.; Uebelhart, D.; Sprott, H. Microcirculation abnormalities in patients with fibromyalgia—Measured by capillary microscopy and laser fluxmetry. Arthritis Res. Ther. 2005, 7, R209–R216. [Google Scholar] [CrossRef]
- Hamilton, J.L.; Nagao, M.; Levine, B.R.; Chen, D.; Olsen, B.R.; Im, H.J. Targeting VEGF and Its Receptors for the Treatment of Osteoarthritis and Associated Pain. J. Bone Miner. Res. 2016, 31, 911–924. [Google Scholar] [CrossRef]
- Russell, F.A.; King, R.; Smillie, S.J.; Kodji, X.; Brain, S.D. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef]
- Tesfay, B.; Karlsson, W.K.; Moreno, R.D.; Hay, D.L.; Hougaard, A. Is calcitonin gene-related peptide a reliable biochemical marker of migraine? Curr. Opin. Neurol. 2022, 35, 343–352. [Google Scholar] [CrossRef]
- Schou, W.S.; Ashina, S.; Amin, F.M.; Goadsby, P.J.; Ashina, M. Calcitonin gene-related peptide and pain: A systematic review. J. Headache Pain 2017, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Sanchez, A.; Brown, C.; Sivan, M.; Talmi, D.; Charalambous, C.; Jones, A.K.P. Are We Any Closer to Understanding How Chronic Pain Develops? A Systematic Search and Critical Narrative Review of Existing Chronic Pain Vulnerability Models. J. Pain Res. 2023, 16, 3145–3166. [Google Scholar]
- Johnson, L.M.; Zautra, A.J.; Davis, M.C. The role of illness uncertainty on coping with fibromyalgia symptoms. Health Psychol. 2006, 25, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Reich, J.W.; Johnson, L.M.; Zautra, A.J.; Davis, M.C. Uncertainty of illness relationships with mental health and coping processes in fibromyalgia patients. J. Behav. Med. 2006, 29, 307–316. [Google Scholar] [CrossRef]
- Reibel, M.D.; Hutti, M.H. The Role of Helplessness in the Appraisal of Illness Uncertainty in Women with Fibromyalgia. Nurs. Sci. Q. 2020, 33, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Araque, A.; Gomez-Castro, J.; Giaquinta-Aranda, A.; Verde, Z.; Torres-Ortega, C. Mishel’s Model of Uncertainty Describing Categories and Subcategories in Fibromyalgia Patients, a Scoping Review. Int. J. Environ. Res. Public Health 2020, 17, 3756. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Mas, A.J.; Carmona, L.; Valverde, M.; Ribas, B.; Navarro, F.; Ortiz, A.M. Prevalence and impact of fibromyalgia on function and quality of life in individuals from the general population: Results from a natiowide study in Spain. Clin. Exp. Rheumatol. 2008, 26, 519–526. [Google Scholar]
- Collado, A.; Gomez, E.; Coscolla, R.; Sunyol, R.; Solé, E.; Rivera, J.; Altarriba, E.; Carbonell, J.; Castells, X. Work, family and social environment in patients with Fibromyalgia in Spain: An epidemiological study: EPIFFAC study. BMC Health Serv. Res. 2014, 14, 513. [Google Scholar] [CrossRef]
- Salgueiro, M.; García-Leiva, J.M.; Ballesteros, J.; Hidalgo, J.; Molina, R.; Calandre, E.P. Validation of a Spanish version of the Revised Fibromyalgia Impact Questionnaire (FIQR). Health Qual. Life Outcomes 2013, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Vargas, A.I.; Roldan-Jimenez, C.; Neblett, R.; Gatchel, R.J. Cross-cultural adaptation and validity of the Spanish central sensitization inventory. Springerplus 2016, 5, 1837. [Google Scholar] [CrossRef]
- Neblett, R.; Hartzell, M.M.; Mayer, T.G.; Cohen, H.; Gatchel, R.J. Establishing Clinically Relevant Severity Levels for the Central Sensitization Inventory. Pain Pract. 2017, 17, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Schuttert, I.; Wolff, A.P.; Schiphorst, R.H.R.; Malmberg, A.G.G.A.; Reneman, M.F.; Timmerman, H. Validity of the Central Sensitization Inventory to Address Human Assumed Central Sensitization: Newly Proposed Clinically Relevant Values and Associations. J. Clin. Med. 2023, 12, 4849. [Google Scholar] [CrossRef] [PubMed]
- Magán, I.; Sanz, J.; García-Vera, M.P. Psychometric properties of a Spanish version of the Beck anxiety inventory (BAI) in general population. Span J. Psychol. 2008, 11, 626–640. [Google Scholar] [CrossRef]
- Do Nascimento, R.L.F.; Fajardo-Bullon, F.; Santos, E.; Landeira-Fernandez, J.; Anunciação, L. Psychometric Properties and Cross-Cultural Invariance of the Beck Depression Inventory-II and Beck Anxiety Inventory among a Representative Sample of Spanish, Portuguese, and Brazilian Undergraduate Students. Int. J. Environ. Res. Public Health 2023, 20, 6009. [Google Scholar] [CrossRef]
- Munguía-Izquierdo, D.; Segura-Jimenez, V.; Camiletti-Moiron, D.; Pulido-Martos, M.; Álvarez-Gallardo, I.C.; Romero, A.; Aparicio, V.A.; Carbonell-Baeza, A.; Delgado-Fernández, M. Multidimensional fatigue inventory: Spanish adaptation and psychometric properties for fibromyalgia patients. The Al-andalus study. Clin. Exp. Rheumatol. 2012, 30, 94–102. [Google Scholar]
- Bakalidou, D.; Krommydas, G.; Abdimioti, T.; Theodorou, P.; Doskas, T.; Fillopoulos, E. The Dimensionality of the Multidimensional Fatigue Inventory (MFI-20) derived from Healthy Adults and Patient Subpopulations: A Challenge for Clinicians. Cureus 2022, 14, e26344. [Google Scholar] [CrossRef]
- Marques, A.P.; Assumpção, A.; Matsutani, L.A.; Bragança Pereira, C.A.; Lage, L. Pain in fibromyalgia and discriminativen power of the instruments: Visual Analog Scale. Dolorimetry and the McGill Pain Questionnaire. Acta Reumatol. Port. 2008, 33, 345–351. [Google Scholar]
- Villanueva-Torrecillas, I.; del Mar Guzman, M.; Javier Toyos, F.; Ariza-Ariza, R.; Navarro, F. Relative efficiency and validity properties of a visual analogue vs a categorical scaled version of the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) index: Spanish versions. Osteoarthr. Cartil. 2004, 12, 225–231. [Google Scholar] [CrossRef]
- Persson, A.L.; Westermark, S.; Merrick, D.; Sjölund, B.H. Validity of electrical stimulus magnitude matching in chronic pain. J. Rehabil. Med. 2009, 41, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Käll, L.B.; Kowalski, J.; Stener-Victorin, E. Assessing pain perception using the Painmatcher in patients with whiplash-associated disorders. J. Rehabil. Med. 2008, 40, 171–1777. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lao, C.; Galiano-Castillo, N.; Cantarero-Villanueva, I.; Martín-Martín, L.; Prados-Olleta, N.; Arroyo-Morales, M. Analysis of pressure pain hypersensitivity, ultrasound image, and quality of life in patients with chronic plantar pain: A preliminary study. Pain Med. 2016, 17, 1530–1541. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Guo, J.Y.; Brown, C.M. Test-retest reliability, repeatability, and sensitivity of an automated deformation-controlled indentation on pressure pain threshold measurement. J. Manip. Physiol. Ther. 2013, 36, 84–90. [Google Scholar] [CrossRef]
- Lindh, C.; Liu, Z.; Welin, M.; Ordeberg, G.; Nyberg, F. Low calcitonin gene-related, peptide-like immunoreactivity in cerebrospinal fluid from chronic pain patients. Neuropeptides 1999, 33, 517–521. [Google Scholar] [CrossRef]
- Muñoz Ladrón de Guevara, C.; Reyes del Paso, G.A.; Fernández Serrano, M.J.; Montoro, C.I. Fibromyalgia Syndrome and Cognitive Decline: The Role of Body Mass Index and Clinical Symptoms. J. Clin. Med. 2022, 11, 3404. [Google Scholar] [CrossRef]
- Sturgeon, J.A.; Darnall, B.D.; Zwickey, H.L.; Wood, L.J.; Hanes, D.A.; Zava, D.T.; Mackey, S.C. Proinflammatory cytokines and DHEA-S in women with fibromyalgia: Impact of psychological distress and menopausal status. J. Pain Res. 2014, 7, 707–716. [Google Scholar] [CrossRef]
- Watt, F.E. Musculoskeletal pain and menopause. Post Reprod. Heal. 2018, 24, 34–43. [Google Scholar] [CrossRef]

| Outcomes | Women Diagnosed with Fibromyalgia (n = 47) | ||
|---|---|---|---|
| Mean ± SD/Frequency (%) | 95% CI | ||
| Age (years) | 56.06 ± 6.41 | [54.18, 57.95] | |
| Height (cm) | 157.83 ± 5.37 | [156.25, 159.41] | |
| Weight (kg) | 68.21 ± 10.09 | [65.24, 71.17] | |
| BMI (kg/cm2) | 27.49 ± 4.59 | [26.14, 28.84] | |
| Menopause status | |||
| Postmenopausal | 38 (80.9) | ||
| Premenopausal | 9 (19.1) | ||
| FIQ-R | 73.24 ± 13.20 | [69.32, 77.16] | |
| CSI | 68.87 ± 11.55 | [65.48, 72.26] | |
| MFI | 79.68 ± 9.79 | [76.81, 82.56] | |
| BAI | 32.60 ± 8.45 | [30.12, 35.08] | |
| VAS (mm) | 74.47 ± 16.66 | [69.58, 79.36] | |
| Electric pain threshold (mA) | 5.75 ± 3.09 | [4.83, 6.67] | |
| Electric pain magnitude (mA) | 11.33 ± 7.89 | [8.99, 13.68] | |
| Pressure pain thresholds (kPa) | |||
| Occiput | D | 1.03 ± 0.42 | [0.82, 1.24] |
| ND | 0.96 ± 0.71 | [0.75, 1.17] | |
| Zygapophyseal joint | D | 1.21 ± 0.82 | [0.97, 1.45] |
| ND | 1.15 ± 0.84 | [0.90, 1.39] | |
| Trapezius | D | 1.07 ± 0.81 | [0.83 1.31] |
| ND | 0.99 ± 0.63 | [0.80, 1.17] | |
| Supraspinatus | D | 1.42 ± 1.13 | [1.09, 1.76] |
| ND | 1.39 ± 0.88 | [1.12, 1.64] | |
| Second rib | D | 0.96 ± 0.53 | [0.80, 1.11] |
| ND | 0.88 ± 0.48 | [0.74, 1.02] | |
| Epicondyle | D | 1.07 ± 0.67 | [0.88, 1.23] |
| ND | 1.05 ± 0.60 | [0.87, 1.22] | |
| Second metacarpal | D | 1.28 ± 0.79 | [1.05, 1.52] |
| ND | 1.15 ± 0.64 | [0.96, 1.34] | |
| Greater trochanter | D | 2.17 ± 1.06 | [1.85, 2.48] |
| ND | 2.22 ± 1.13 | [1.98, 2.55] | |
| Gluteus | D | 2.09 ± 1.67 | [1.59, 2.59] |
| ND | 1.97 ± 1.33 | [1.58, 2.36] | |
| Knee | D | 1.81 ± 1.17 | [1.46, 2.15] |
| ND | 1.99 ± 1.11 | [1.66, 2.32] | |
| Anterior tibialis | D | 1.99 ± 1.33 | [1.60, 2.38] |
| ND | 1.97 ± 1.18 | [1.62, 2.32] | |
| VEGF (pg/mL) | 354.29 ± 269.69 | [275.11, 433.48] | |
| CGRP (pg/mL) | 36.96 ± 5.83 | [35.25, 38.67] | |
| Variable | Women Diagnosed with Fibromyalgia (n = 47) | |||
|---|---|---|---|---|
| Serum VEGF Levels | ||||
| β | 95% CI | p-Value | ||
| FIQ-R | −2.929 | [−9.511, 3.653] | 0.374 | |
| CSI | −7.586 | [−15.607, 0.435] | 0.063 | |
| MFI | −6.163 | [−14.242, 1.920] | 0.131 | |
| BAI | −6.323 | [−15.810, 3.164] | 0.186 | |
| VAS (mm) | −0.232 | [−19.276, 18.813] | 0.981 | |
| Electric pain threshold (mA) | 130.464 | [−56.148, 317.075] | 0.166 | |
| Electric pain magnitude (mA) | 131.936 | [−21.741, 285.613] | 0.090 | |
| Pressure pain thresholds (kPa) | ||||
| Occiput | D | 81.418 | [−45.012, 207.849] | 0.201 |
| ND | 105.729 | [−13.284, 224.741] | 0.080 | |
| Zygapophyseal joint | D | 41.557 | [−59.295, 142.408] | 0.410 |
| ND | 77.398 | [−17.147, 171.942] | 0.106 | |
| Trapezius | D | 76.786 | [−29.710, 183.283] | 0.153 |
| ND | 153.418 | [12.877, 293.958] | 0.033 * | |
| Supraspinatus | D | 67.588 | [−47.595, 182.771] | 0.243 |
| ND | 85.281 | [−7.209, 177.771] | 0.070 | |
| Second rib | D | 82.572 | [−75.866, 241.011] | 0.299 |
| ND | 44.153 | [−136.470, 224.776] | 0.624 | |
| Epicondyle | D | 94.297 | [−35.924, 224.517] | 0.151 |
| ND | 124.098 | [−22.241, 270.437] | 0.094 | |
| Second metacarpal | D | 46.024 | [−55.289, 147.336] | 0.364 |
| ND | 174.676 | [47.910, 301.442] | 0.008 * | |
| Greater trochanter | D | 29.501 | [−55.326, 114.329] | 0.486 |
| ND | 62.310 | [−18.607, 143.228] | 0.128 | |
| Gluteus | D | 92.195 | [−32.859, 217.248] | 0.144 |
| ND | 52.960 | [−11.039, 116.960] | 0.102 | |
| Knee | D | 37.270 | [−36.219, 110.758] | 0.312 |
| ND | 68.053 | [−6.586, 142.691] | 0.073 | |
| Anterior tibialis | D | 115.080 | [0.510, 229.650] | 0.049 * |
| ND | 57.224 | [−12.185, 126.634] | 0.104 | |
| Variable | Women Diagnosed with Fibromyalgia (n = 47) | |||
|---|---|---|---|---|
| Serum CGRP Levels | ||||
| β | 95% CI | p-Value | ||
| FIQ-R | −0.101 | [−0.241, 0.039] | 0.152 | |
| CSI | −0.014 | [−0.173, 0.146] | 0.864 | |
| MFI | −0.085 | [−0.263, 0.092] | 0.339 | |
| BAI | −0.035 | [−0.244,0.174] | 0.737 | |
| VAS (mm) | 0.064 | [−0.46, 0.175] | 0.248 | |
| Electric pain threshold (mA) | −2.680 | [−6.701, 1.342] | 0.186 | |
| Electric pain magnitude (mA) | 0.473 | [−2.947, 3.893] | 0.781 | |
| Pressure pain thresholds (kPa) | ||||
| Occiput | D | 0.607 | [−2.173, 3.386] | 0.622 |
| ND | 0.190 | [−2.477, 2.857] | 0.887 | |
| Zygapophyseal joint | D | −0.222 | [−2.418, 1.973] | 0.839 |
| ND | −0.847 | [−2.938, 1.245] | 0.419 | |
| Trapezius | D | −0.501 | [−2.854, 1.852] | 0.669 |
| ND | −1.166 | [−4.135, 1.803] | 0.433 | |
| Supraspinatus | D | −0.684 | [−3.204, 1.837] | 0.587 |
| ND | −0.014 | [−2.222, 1.935] | 0.890 | |
| Second rib | D | −1.536 | [−4.970, 1.899] | 0.372 |
| ND | −1.759 | [−5.634, 2.116] | 0.365 | |
| Epicondyle | D | −0.327 | [−3.210, 2.555] | 0.820 |
| ND | −1.216 | [−4.274, 1.843] | 0.427 | |
| Second metacarpal | D | 1.222 | [−1.021, 3.466] | 0.277 |
| ND | 0.638 | [−2.336, 3.611] | 0.667 | |
| Greater trochanter | D | 0.813 | [−0.978, 2.603] | 0.364 |
| ND | 0.153 | [−1.613, 31.919] | 0.862 | |
| Gluteus | D | −0.255 | [−3.026, 2.516] | 0.854 |
| ND | 0.159 | [−1.268, 1.586] | 0.823 | |
| Knee | D | 0.179 | [−1.428, 1.785] | 0.823 |
| ND | 0.062 | [−1.614, 1.739] | 0.940 | |
| Anterior tibialis | D | −0.384 | [−2.975, 2.207] | 0.766 |
| ND | −0.212 | [−1.759, 1.335] | 0.783 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tapia-Haro, R.M.; Molina, F.; Rus, A.; Casas-Barragán, A.; Correa-Rodríguez, M.; Aguilar-Ferrándiz, M.E. Serum VEGF and CGRP Biomarkers: Relationships with Pain Intensity, Electric Pain, Pressure Pain Threshold, and Clinical Symptoms in Fibromyalgia—An Observational Study. Int. J. Mol. Sci. 2023, 24, 15533. https://doi.org/10.3390/ijms242115533
Tapia-Haro RM, Molina F, Rus A, Casas-Barragán A, Correa-Rodríguez M, Aguilar-Ferrándiz ME. Serum VEGF and CGRP Biomarkers: Relationships with Pain Intensity, Electric Pain, Pressure Pain Threshold, and Clinical Symptoms in Fibromyalgia—An Observational Study. International Journal of Molecular Sciences. 2023; 24(21):15533. https://doi.org/10.3390/ijms242115533
Chicago/Turabian StyleTapia-Haro, Rosa Mª, Francisco Molina, Alma Rus, Antonio Casas-Barragán, María Correa-Rodríguez, and Mª Encarnación Aguilar-Ferrándiz. 2023. "Serum VEGF and CGRP Biomarkers: Relationships with Pain Intensity, Electric Pain, Pressure Pain Threshold, and Clinical Symptoms in Fibromyalgia—An Observational Study" International Journal of Molecular Sciences 24, no. 21: 15533. https://doi.org/10.3390/ijms242115533
APA StyleTapia-Haro, R. M., Molina, F., Rus, A., Casas-Barragán, A., Correa-Rodríguez, M., & Aguilar-Ferrándiz, M. E. (2023). Serum VEGF and CGRP Biomarkers: Relationships with Pain Intensity, Electric Pain, Pressure Pain Threshold, and Clinical Symptoms in Fibromyalgia—An Observational Study. International Journal of Molecular Sciences, 24(21), 15533. https://doi.org/10.3390/ijms242115533