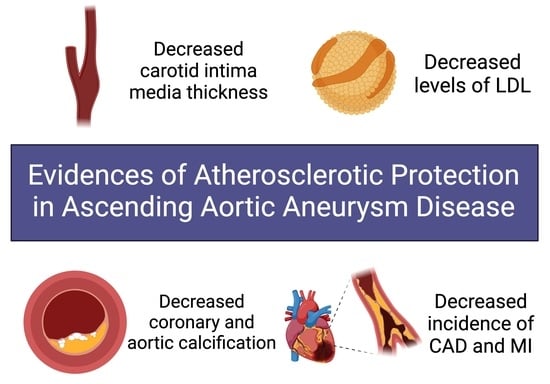
Evidence Accumulates: Patients with Ascending Aneurysms Are Strongly Protected from Atherosclerotic Disease
Abstract
:1. Introduction
2. Embryology of the Aorta
3. Our Investigations
3.1. Intimal Medial Thickness
3.2. Lipid Profiles
3.3. Coronary Artery and Aortic Calcification
3.4. CAD/Total Protection from MI
4. Aortic Histology
5. Discussion: Potential Mechanisms of Anti-Atherogenic Protection
5.1. Aortic Vascular Smooth Muscle Cells
5.2. Phenotypic Switch Defect in Thoracic Aortopathy Discourages Atherosclerosis
5.3. MMP/TMP Dysregulation Accompanies VSMC Disturbances
5.4. TGF- Provides Anti-Atherogenic Contribution
5.5. Hemodynamic Changes
6. Conclusions/Future Steps/Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zarins, C.K.; Xu, C.; Glagov, S. Atherosclerotic enlargement of the human abdominal aorta. Atherosclerosis 2001, 155, 157–164. [Google Scholar] [CrossRef]
- Singh, K.; Bonaa, K.H.; Jacobsen, B.K.; Bjork, L.; Solberg, S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: The Tromso Study. Am. J. Epidemiol. 2001, 154, 236–244. [Google Scholar] [CrossRef]
- Tung, W.S.; Lee, J.K.; Thompson, R.W. Simultaneous analysis of 1176 gene products in normal human aorta and abdominal aortic aneurysms using a membrane-based complementary DNA expression array. J. Vasc. Surg. 2001, 34, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Kuhlencordt, P.J.; Gyurko, R.; Han, F.; Scherrer-Crosbie, M.; Aretz, T.H.; Hajjar, R.; Picard, M.H.; Huang, P.L. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation 2001, 104, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Biddinger, A.; Rocklin, M.; Coselli, J.; Milewicz, D.M. Familial thoracic aortic dilatations and dissections: A case control study. J. Vasc. Surg. 1997, 25, 506–511. [Google Scholar] [CrossRef]
- Ostberg, N.P.; Zafar, M.A.; Ziganshin, B.A.; Elefteriades, J.A. The Genetics of Thoracic Aortic Aneurysms and Dissection: A Clinical Perspective. Biomolecules 2020, 10, 182. [Google Scholar] [CrossRef]
- Schmidt, J.; Sunesen, K.; Kornum, J.B.; Duhaut, P.; Thomsen, R.W. Predictors for pathologically confirmed aortitis after resection of the ascending aorta: A 12-year Danish nationwide population-based cross-sectional study. Arthritis Res. Ther. 2011, 13, R87. [Google Scholar] [CrossRef]
- Evans, J.M.; O’Fallon, W.M.; Hunder, G.G. Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis. A population-based study. Ann. Intern. Med. 1995, 122, 502–507. [Google Scholar] [CrossRef]
- Roberts, W.C.; Barbin, C.M.; Weissenborn, M.R.; Ko, J.M.; Henry, A.C. Syphilis as a Cause of Thoracic Aortic Aneurysm. Am. J. Cardiol. 2015, 116, 1298–1303. [Google Scholar] [CrossRef]
- Elefteriades, J.A.; Ziganshin, B.A.; Halperin, J.L. Diseases of the Aorta. In Fuster and Hurst’s the Heart, 15e; Fuster, V., Narula, J., Vaishnava, P., Leon, M.B., Callans, D.J., Rumsfeld, J., Poppas, A., Eds.; McGraw-Hill Education: New York, NY, USA, 2022. [Google Scholar]
- Ruddy, J.M.; Jones, J.A.; Spinale, F.G.; Ikonomidis, J.S. Regional heterogeneity within the aorta: Relevance to aneurysm disease. J. Thorac. Cardiovasc. Surg. 2008, 136, 1123–1130. [Google Scholar] [CrossRef]
- Elefteriades, J.A.; Farkas, E.A. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J. Am. Coll. Cardiol. 2010, 55, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Ruddy, J.M.; Jones, J.A.; Ikonomidis, J.S. Pathophysiology of thoracic aortic aneurysm (TAA): Is it not one uniform aorta? Role of embryologic origin. Prog. Cardiovasc. Dis. 2013, 56, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Grewal, N.; Gittenberger-de Groot, A.C.; Lindeman, J.H.; Klautz, A.; Driessen, A.; Klautz, R.J.M.; Poelmann, R.E. Normal and abnormal development of the aortic valve and ascending aortic wall: A comprehensive overview of the embryology and pathology of the bicuspid aortic valve. Ann. Cardiothorac. Surg. 2022, 11, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Halloran, B.G.; Davis, V.A.; McManus, B.M.; Lynch, T.G.; Baxter, B.T. Localization of aortic disease is associated with intrinsic differences in aortic structure. J. Surg. Res. 1995, 59, 17–22. [Google Scholar] [CrossRef]
- Bots, M.L.; Grobbee, D.E. Intima media thickness as a surrogate marker for generalised atherosclerosis. Cardiovasc. Drugs Ther. 2002, 16, 341–351. [Google Scholar] [CrossRef]
- Crouse, J.R.; Goldbourt, U.; Evans, G.; Pinsky, J.; Sharrett, A.R.; Sorlie, P.; Riley, W.; Heiss, G. Risk factors and segment-specific carotid arterial enlargement in the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke 1996, 27, 69–75. [Google Scholar] [CrossRef]
- Bots, M.L.; Hoes, A.W.; Koudstaal, P.J.; Hofman, A.; Grobbee, D.E. Common carotid intima-media thickness and risk of stroke and myocardial infarction: The Rotterdam Study. Circulation 1997, 96, 1432–1437. [Google Scholar] [CrossRef]
- O’Leary, D.H.; Polak, J.F.; Kronmal, R.A.; Manolio, T.A.; Burke, G.L.; Wolfson, S.K., Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N. Engl. J. Med. 1999, 340, 14–22. [Google Scholar] [CrossRef]
- Urbina, E.M.; Srinivasan, S.R.; Tang, R.; Bond, M.G.; Kieltyka, L.; Berenson, G.S.; Bogalusa Heart, S. Impact of multiple coronary risk factors on the intima-media thickness of different segments of carotid artery in healthy young adults (The Bogalusa Heart Study). Am. J. Cardiol. 2002, 90, 953–958. [Google Scholar] [CrossRef]
- Salonen, J.T.; Salonen, R. Ultrasound B-mode imaging in observational studies of atherosclerotic progression. Circulation 1993, 87, II56–II65. [Google Scholar]
- Johnsen, S.H.; Mathiesen, E.B.; Joakimsen, O.; Stensland, E.; Wilsgaard, T.; Lochen, M.L.; Njolstad, I.; Arnesen, E. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: A 6-year follow-up study of 6226 persons: The Tromso Study. Stroke 2007, 38, 2873–2880. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Corrado, E.; Coppola, G.; Muratori, I.; Novo, G.; Novo, S. Prediction of cardio- and cerebro-vascular events in patients with subclinical carotid atherosclerosis and low HDL-cholesterol. Atherosclerosis 2008, 200, 389–395. [Google Scholar] [CrossRef]
- Novo, S.; Peritore, A.; Trovato, R.L.; Guarneri, F.P.; Di Lisi, D.; Muratori, I.; Novo, G. Preclinical atherosclerosis and metabolic syndrome increase cardio- and cerebrovascular events rate: A 20-year follow up. Cardiovasc. Diabetol. 2013, 12, 155. [Google Scholar] [CrossRef]
- Stein, J.H.; Fraizer, M.C.; Aeschlimann, S.E.; Nelson-Worel, J.; McBride, P.E.; Douglas, P.S. Vascular age: Integrating carotid intima-media thickness measurements with global coronary risk assessment. Clin. Cardiol. 2004, 27, 388–392. [Google Scholar] [CrossRef]
- Stein, J.H.; Korcarz, C.E.; Hurst, R.T.; Lonn, E.; Kendall, C.B.; Mohler, E.R.; Najjar, S.S.; Rembold, C.M.; Post, W.S. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J. Am. Soc. Echocardiogr. 2008, 21, 93–111, quiz 189–190. [Google Scholar] [CrossRef]
- Ali, Y.S.; Rembold, K.E.; Weaver, B.; Wills, M.B.; Tatar, S.; Ayers, C.R.; Rembold, C.M. Prediction of major adverse cardiovascular events by age-normalized carotid intimal medial thickness. Atherosclerosis 2006, 187, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Gepner, A.D.; Keevil, J.G.; Wyman, R.A.; Korcarz, C.E.; Aeschlimann, S.E.; Busse, K.L.; Stein, J.H. Use of carotid intima-media thickness and vascular age to modify cardiovascular risk prediction. J. Am. Soc. Echocardiogr. 2006, 19, 1170–1174. [Google Scholar] [CrossRef]
- Ludwig, M.; von Petzinger-Kruthoff, A.; von Buquoy, M.; Stumpe, K.O. Intima-Media-Dicke der Karotisarterien: Fruher Indikator fur Arteriosklerose und therapeutischer Endpunkt. [Intima media thickness of the carotid arteries: Early pointer to arteriosclerosis and therapeutic endpoint]. Ultraschall Med. 2003, 24, 162–174. [Google Scholar] [CrossRef]
- Baldassarre, D.; Amato, M.; Bondioli, A.; Sirtori, C.R.; Tremoli, E. Carotid artery intima-media thickness measured by ultrasonography in normal clinical practice correlates well with atherosclerosis risk factors. Stroke 2000, 31, 2426–2430. [Google Scholar] [CrossRef]
- Poredos, P. Intima-media thickness: Indicator of cardiovascular risk and measure of the extent of atherosclerosis. Vasc. Med. 2004, 9, 46–54. [Google Scholar] [CrossRef]
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvall, M.; Sitzer, M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation 2007, 115, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Chambless, L.E.; Heiss, G.; Folsom, A.R.; Rosamond, W.; Szklo, M.; Sharrett, A.R.; Clegg, L.X. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: The Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am. J. Epidemiol. 1997, 146, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Greenland, P.; Alpert, J.S.; Beller, G.A.; Benjamin, E.J.; Budoff, M.J.; Fayad, Z.A.; Foster, E.; Hlatky, M.A.; Hodgson, J.M.; Kushner, F.G.; et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2010, 56, e50–e103. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.F.; O’Leary, D.H.; Kronmal, R.A.; Wolfson, S.K.; Bond, M.G.; Tracy, R.P.; Gardin, J.M.; Kittner, S.J.; Price, T.R.; Savage, P.J. Sonographic evaluation of carotid artery atherosclerosis in the elderly: Relationship of disease severity to stroke and transient ischemic attack. Radiology 1993, 188, 363–370. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, D.H.; Polak, J.F.; Kronmal, R.A.; Savage, P.J.; Borhani, N.O.; Kittner, S.J.; Tracy, R.; Gardin, J.M.; Price, T.R.; Furberg, C.D. Thickening of the carotid wall. A marker for atherosclerosis in the elderly? Cardiovascular Health Study Collaborative Research Group. Stroke 1996, 27, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.F.; Szklo, M.; O’Leary, D.H. Carotid Intima-Media Thickness Score, Positive Coronary Artery Calcium Score, and Incident Coronary Heart Disease: The Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2017, 6, e004612. [Google Scholar] [CrossRef]
- Kim, G.H.; Youn, H.J. Is Carotid Artery Ultrasound Still Useful Method for Evaluation of Atherosclerosis? Korean Circ. J. 2017, 47, 1–8. [Google Scholar] [CrossRef]
- Saba, L.; Jamthikar, A.; Gupta, D.; Khanna, N.N.; Viskovic, K.; Suri, H.S.; Gupta, A.; Mavrogeni, S.; Turk, M.; Laird, J.R.; et al. Global perspective on carotid intima-media thickness and plaque: Should the current measurement guidelines be revisited? Int. Angiol. 2019, 38, 451–465. [Google Scholar] [CrossRef]
- Hung, A.; Zafar, M.; Mukherjee, S.; Tranquilli, M.; Scoutt, L.M.; Elefteriades, J.A. Carotid intima-media thickness provides evidence that ascending aortic aneurysm protects against systemic atherosclerosis. Cardiology 2012, 123, 71–77. [Google Scholar] [CrossRef]
- Norrgard, O.; Angquist, K.A.; Dahlen, G. High concentrations of Lp(a) lipoprotein in serum are common among patients with abdominal aortic aneurysms. Int. Angiol. 1988, 7, 46–49. [Google Scholar]
- Papagrigorakis, E.; Iliopoulos, D.; Asimacopoulos, P.J.; Safi, H.J.; Weilbaecher, D.J.; Ghazzaly, K.G.; Nava, M.L.; Gaubatz, J.W.; Morrisett, J.D. Lipoprotein(a) in plasma, arterial wall, and thrombus from patients with aortic aneurysm. Clin. Genet. 1997, 52, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Schillinger, M.; Domanovits, H.; Ignatescu, M.; Exner, M.; Bayegan, K.; Sedivy, R.; Polterauer, P.; Laggner, A.N.; Minar, E.; Kostner, K. Lipoprotein (a) in patients with aortic aneurysmal disease. J. Vasc. Surg. 2002, 36, 25–30. [Google Scholar] [CrossRef]
- Naydeck, B.L.; Sutton-Tyrrell, K.; Schiller, K.D.; Newman, A.B.; Kuller, L.H. Prevalence and risk factors for abdominal aortic aneurysms in older adults with and without isolated systolic hypertension. Am. J. Cardiol. 1999, 83, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Cushing, G.L.; Gaubatz, J.W.; Nava, M.L.; Burdick, B.J.; Bocan, T.M.; Guyton, J.R.; Weilbaecher, D.; DeBakey, M.E.; Lawrie, G.M.; Morrisett, J.D. Quantitation and localization of apolipoproteins [a] and B in coronary artery bypass vein grafts resected at re-operation. Arteriosclerosis 1989, 9, 593–603. [Google Scholar] [CrossRef]
- Kostner, G.M.; Avogaro, P.; Cazzolato, G.; Marth, E.; Bittolo-Bon, G.; Qunici, G.B. Lipoprotein Lp(a) and the risk for myocardial infarction. Atherosclerosis 1981, 38, 51–61. [Google Scholar] [CrossRef]
- Kostner, K.M.; Oberbauer, R.; Hoffmann, U.; Stefenelli, T.; Maurer, G.; Watschinger, B. Urinary excretion of apo(a) in patients after kidney transplantation. Nephrol. Dial. Transplant. 1997, 12, 2673–2678. [Google Scholar] [CrossRef]
- Murai, A.; Miyahara, T.; Fujimoto, N.; Matsuda, M.; Kameyama, M. Lp(a) lipoprotein as a risk factor for coronary heart disease and cerebral infarction. Atherosclerosis 1986, 59, 199–204. [Google Scholar] [CrossRef]
- Berg, K.; Dahlen, G.; Frick, M.H. Lp(a) lipoprotein and pre-beta1-lipoprotein in patients with coronary heart disease. Clin. Genet. 1974, 6, 230–235. [Google Scholar] [CrossRef]
- Armstrong, V.W.; Cremer, P.; Eberle, E.; Manke, A.; Schulze, F.; Wieland, H.; Kreuzer, H.; Seidel, D. The association between serum Lp(a) concentrations and angiographically assessed coronary atherosclerosis. Dependence on serum LDL levels. Atherosclerosis 1986, 62, 249–257. [Google Scholar] [CrossRef]
- Weininger, G.; Ostberg, N.; Shang, M.; Zafar, M.; Ziganshin, B.A.; Liu, S.; Erben, Y.; Elefteriades, J.A. Lipid profiles help to explain protection from systemic atherosclerosis in patients with ascending aortic aneurysm. J. Thorac. Cardiovasc. Surg. 2022, 163, e129–e132. [Google Scholar] [CrossRef]
- Kita, T.; Kume, N.; Minami, M.; Hayashida, K.; Murayama, T.; Sano, H.; Moriwaki, H.; Kataoka, H.; Nishi, E.; Horiuchi, H.; et al. Role of oxidized LDL in atherosclerosis. Ann. N. Y. Acad. Sci. 2001, 947, 199–205, discussion 205–196. [Google Scholar] [CrossRef] [PubMed]
- Steinbrecher, U.P.; Zhang, H.F.; Lougheed, M. Role of oxidatively modified LDL in atherosclerosis. Free Radic. Biol. Med. 1990, 9, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Morabito, G.; Urban, L.; Ioannone, F.; Serafini, M. Oxidative stress in atherosclerosis development: The central role of LDL and oxidative burst. Endocr. Metab. Immune Disord. Drug Targets 2012, 12, 351–360. [Google Scholar] [CrossRef]
- Saigusa, T.; Izawa, A.; Miura, T.; Ebisawa, S.; Shiba, Y.; Miyashita, Y.; Tomita, T.; Koyama, J.; Fukui, D.; Takano, T.; et al. Low levels of high-density lipoprotein cholesterol predict the presence of coronary artery disease in patients with aortic aneurysms. Angiology 2014, 65, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Allison, M.A.; Criqui, M.H.; Wright, C.M. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Solberg, L.A.; Eggen, D.A. Localization and sequence of development of atherosclerotic lesions in the carotid and vertebral arteries. Circulation 1971, 43, 711–724. [Google Scholar] [CrossRef]
- Rifkin, R.D.; Parisi, A.F.; Folland, E. Coronary calcification in the diagnosis of coronary artery disease. Am. J. Cardiol. 1979, 44, 141–147. [Google Scholar] [CrossRef]
- Sangiorgi, G.; Rumberger, J.A.; Severson, A.; Edwards, W.D.; Gregoire, J.; Fitzpatrick, L.A.; Schwartz, R.S. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: A histologic study of 723 coronary artery segments using nondecalcifying methodology. J. Am. Coll. Cardiol. 1998, 31, 126–133. [Google Scholar] [CrossRef]
- Doherty, T.M.; Detrano, R.C.; Mautner, S.L.; Mautner, G.C.; Shavelle, R.M. Coronary calcium: The good, the bad, and the uncertain. Am. Heart J. 1999, 137, 806–814. [Google Scholar] [CrossRef]
- Doherty, T.M.; Fitzpatrick, L.A.; Shaheen, A.; Rajavashisth, T.B.; Detrano, R.C. Genetic determinants of arterial calcification associated with atherosclerosis. Mayo Clin. Proc. 2004, 79, 197–210. [Google Scholar] [CrossRef]
- Watson, K.E. Pathophysiology of coronary calcification. J. Cardiovasc. Risk 2000, 7, 93–97. [Google Scholar] [CrossRef]
- Kuller, L.H.; Matthews, K.A.; Sutton-Tyrrell, K.; Edmundowicz, D.; Bunker, C.H. Coronary and aortic calcification among women 8 years after menopause and their premenopausal risk factors: The healthy women study. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2189–2198. [Google Scholar] [CrossRef]
- McCullough, P.A.; Soman, S. Cardiovascular calcification in patients with chronic renal failure: Are we on target with this risk factor? Kidney Int. Suppl. 2004, 66, S18–S24. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.B.; Naydeck, B.L.; Sutton-Tyrrell, K.; Edmundowicz, D.; O’Leary, D.; Kronmal, R.; Burke, G.L.; Kuller, L.H. Relationship between coronary artery calcification and other measures of subclinical cardiovascular disease in older adults. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1674–1679. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.J.; Raggi, P.; Schisterman, E.; Berman, D.S.; Callister, T.Q. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology 2003, 228, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.R.; Partridge, J. Coronary calcification score: The coronary-risk impact factor. Lancet 2004, 363, 557–559. [Google Scholar] [CrossRef]
- Danielsen, R.; Sigvaldason, H.; Thorgeirsson, G.; Sigfusson, N. Predominance of aortic calcification as an atherosclerotic manifestation in women: The Reykjavik study. J. Clin. Epidemiol. 1996, 49, 383–387. [Google Scholar] [CrossRef]
- Iribarren, C.; Sidney, S.; Sternfeld, B.; Browner, W.S. Calcification of the aortic arch: Risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA 2000, 283, 2810–2815. [Google Scholar] [CrossRef]
- Li, J.; Galvin, H.K.; Johnson, S.C.; Langston, C.S.; Sclamberg, J.; Preston, C.A. Aortic calcification on plain chest radiography increases risk for coronary artery disease. Chest 2002, 121, 1468–1471. [Google Scholar] [CrossRef]
- Symeonidis, G.; Papanas, N.; Giannakis, I.; Mavridis, G.; Lakasas, G.; Kyriakidis, G.; Artopoulos, I. Gravity of aortic arch calcification as evaluated in adult Greek patients. Int. Angiol. 2002, 21, 233–236. [Google Scholar]
- Takasu, J.; Mao, S.; Budoff, M.J. Aortic atherosclerosis detected with electron-beam CT as a predictor of obstructive coronary artery disease. Acad. Radiol. 2003, 10, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Shavelle, D.; Takasu, J.; Lu, B.; Mao, S.S.; Fischer, H.; Budoff, M.J. Valvular and thoracic aortic calcium as a marker of the extent and severity of angiographic coronary artery disease. Am. Heart J. 2003, 146, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Takasu, J.; Yamamoto, R.; Yokoyama, K.; Taguchi, R.; Itani, Y.; Imai, H.; Koizumi, T.; Nomoto, K.; Sato, N.; et al. Assessment of aortic atherosclerosis and carotid atherosclerosis in coronary artery disease. Jpn. Circ. J. 2000, 64, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, S.; Ropers, D.; Mohlenkamp, S.; Schmermund, A.; Muschiol, G.; Groth, J.; Kusus, M.; Regenfus, M.; Daniel, W.G.; Erbel, R.; et al. Variability of repeated coronary artery calcium measurements by electron beam tomography. Am. J. Cardiol. 2001, 87, 210–213, A218. [Google Scholar] [CrossRef] [PubMed]
- Janowitz, W.R.; Agatston, A.S.; Kaplan, G.; Viamonte, M., Jr. Differences in prevalence and extent of coronary artery calcium detected by ultrafast computed tomography in asymptomatic men and women. Am. J. Cardiol. 1993, 72, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, D.; Toulgaridis, T.; Davlouros, P.; Christodoulou, J.; Sitafidis, G.; Hahalis, G.; Vagenakis, A.G. Prognostic significance of coronary artery calcium in asymptomatic subjects with usual cardiovascular risk. Am. Heart J. 2003, 145, 542–548. [Google Scholar] [CrossRef]
- Jayalath, R.W.; Mangan, S.H.; Golledge, J. Aortic calcification. Eur. J. Vasc. Endovasc. Surg. 2005, 30, 476–488. [Google Scholar] [CrossRef]
- O’Malley, P.G.; Taylor, A.J.; Jackson, J.L.; Doherty, T.M.; Detrano, R.C. Prognostic value of coronary electron-beam computed tomography for coronary heart disease events in asymptomatic populations. Am. J. Cardiol. 2000, 85, 945–948. [Google Scholar] [CrossRef]
- Achneck, H.; Modi, B.; Shaw, C.; Rizzo, J.; Albornoz, G.; Fusco, D.; Elefteriades, J. Ascending thoracic aneurysms are associated with decreased systemic atherosclerosis. Chest 2005, 128, 1580–1586. [Google Scholar] [CrossRef]
- Islamoglu, F.; Atay, Y.; Can, L.; Kara, E.; Ozbaran, M.; Yuksel, M.; Buket, S. Diagnosis and treatment of concomitant aortic and coronary disease: A retrospective study and brief review. Tex. Heart Inst. J. 1999, 26, 182–188. [Google Scholar]
- Agmon, Y.; Khandheria, B.K.; Meissner, I.; Schwartz, G.L.; Sicks, J.D.; Fought, A.J.; O’Fallon, W.M.; Wiebers, D.O.; Tajik, A.J. Is aortic dilatation an atherosclerosis-related process? Clinical, laboratory, and transesophageal echocardiographic correlates of thoracic aortic dimensions in the population with implications for thoracic aortic aneurysm formation. J. Am. Coll. Cardiol. 2003, 42, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Suwa, S.; Fujiwara, Y.; Inoue, K.; Mineda, Y.; Ohta, H.; Tokano, T.; Nakata, Y. Incidence and severity of coronary artery disease in patients with acute aortic dissection: Comparison with abdominal aortic aneurysm and arteriosclerosis obliterans. J. Cardiol. 2001, 37, 165–171. [Google Scholar] [PubMed]
- Nakashima, Y.; Kurozumi, T.; Sueishi, K.; Tanaka, K. Dissecting aneurysm: A clinicopathologic and histopathologic study of 111 autopsied cases. Hum. Pathol. 1990, 21, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Yeager, R.A.; Weigel, R.M.; Murphy, E.S.; McConnell, D.B.; Sasaki, T.M.; Vetto, R.M. Application of clinically valid cardiac risk factors to aortic aneurysm surgery. Arch. Surg. 1986, 121, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Langanay, T.; Valla, J.; Le Du, J.; Verhoye, J.P.; Leguerrier, A.; Lelong, B.; Menestret, P.; Rioux, C.; Logeais, Y. Insuffisance coronaire chez les patients ayant un anevrysme de l’aorte abdominale. A propos d’une serie consecutive de 172 operes. [Coronary artery disease in patients with aortic abdominal aneurysm. Apropos of a consecutive series of 172 cases]. Arch. Mal. Coeur Vaiss. 1996, 89, 211–218. [Google Scholar]
- Ruby, S.T.; Whittemore, A.D.; Couch, N.P.; Collins, J.J.; Cohn, L.; Shemin, R.; Mannick, J.A. Coronary artery disease in patients requiring abdominal aortic aneurysm repair. Selective use of a combined operation. Ann. Surg. 1985, 201, 758–764. [Google Scholar] [CrossRef]
- Kishi, K.; Ito, S.; Hiasa, Y. Risk factors and incidence of coronary artery lesions in patients with abdominal aortic aneurysms. Intern. Med. 1997, 36, 384–388. [Google Scholar] [CrossRef]
- Chau, K.; Elefteriades, J.A. Ascending thoracic aortic aneurysms protect against myocardial infarctions. Int. J. Angiol. 2014, 23, 177–182. [Google Scholar] [CrossRef]
- Dolmaci, O.B.; El Mathari, S.; Driessen, A.H.G.; Klautz, R.J.M.; Poelmann, R.E.; Lindeman, J.H.N.; Grewal, N. Are Thoracic Aortic Aneurysm Patients at Increased Risk for Cardiovascular Diseases? J. Clin. Med. 2022, 12, 272. [Google Scholar] [CrossRef]
- Jackson, V.; Eriksson, M.J.; Caidahl, K.; Eriksson, P.; Franco-Cereceda, A. Ascending aortic dilatation is rarely associated with coronary artery disease regardless of aortic valve morphology. J. Thorac. Cardiovasc. Surg. 2014, 148, 2973–2980.e2971. [Google Scholar] [CrossRef]
- Khoury, Z.; Gottlieb, S.; Stern, S.; Keren, A. Frequency and distribution of atherosclerotic plaques in the thoracic aorta as determined by transesophageal echocardiography in patients with coronary artery disease. Am. J. Cardiol. 1997, 79, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Grewal, N.; Dolmaci, O.; Jansen, E.; Klautz, R.; Driessen, A.; Lindeman, J.; Poelmann, R.E. Are acute type A aortic dissections atherosclerotic? Front. Cardiovasc. Med. 2022, 9, 1032755. [Google Scholar] [CrossRef] [PubMed]
- Hashiyama, N.; Goda, M.; Uchida, K.; Isomatsu, Y.; Suzuki, S.; Mo, M.; Nishida, T.; Masuda, M. Stanford type B aortic dissection is more frequently associated with coronary artery atherosclerosis than type A. J. Cardiothorac. Surg. 2018, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Leone, O.; Corsini, A.; Pacini, D.; Corti, B.; Lorenzini, M.; Laus, V.; Foa, A.; Bacchi Reggiani, M.L.; Di Marco, L.; Rapezzi, C. The complex interplay among atherosclerosis, inflammation, and degeneration in ascending thoracic aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2020, 160, 1434–1443.e1436. [Google Scholar] [CrossRef] [PubMed]
- Leone, O.; Pacini, D.; Foa, A.; Corsini, A.; Agostini, V.; Corti, B.; Di Marco, L.; Leone, A.; Lorenzini, M.; Reggiani, L.B.; et al. Redefining the histopathologic profile of acute aortic syndromes: Clinical and prognostic implications. J. Thorac. Cardiovasc. Surg. 2018, 156, 1776–1785.e1776. [Google Scholar] [CrossRef]
- Albini, P.T.; Segura, A.M.; Liu, G.; Minard, C.G.; Coselli, J.S.; Milewicz, D.M.; Shen, Y.H.; LeMaire, S.A. Advanced atherosclerosis is associated with increased medial degeneration in sporadic ascending aortic aneurysms. Atherosclerosis 2014, 232, 361–368. [Google Scholar] [CrossRef]
- Stejskal, V.; Karalko, M.; Krbal, L. Histopathological findings of diseased ascending aortae with clinicopathological correlation—A single-centre study of 160 cases. Pathol. Res. Pract. 2023, 246, 154526. [Google Scholar] [CrossRef]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef]
- He, X.; Lian, Z.; Yang, Y.; Wang, Z.; Fu, X.; Liu, Y.; Li, M.; Tian, J.; Yu, T.; Xin, H. Long Non-coding RNA PEBP1P2 Suppresses Proliferative VSMCs Phenotypic Switching and Proliferation in Atherosclerosis. Mol. Ther. Nucleic Acids 2020, 22, 84–98. [Google Scholar] [CrossRef]
- Shanahan, C.M.; Crouthamel, M.H.; Kapustin, A.; Giachelli, C.M. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ. Res. 2011, 109, 697–711. [Google Scholar] [CrossRef]
- Chin, D.D.; Poon, C.; Wang, J.; Joo, J.; Ong, V.; Jiang, Z.; Cheng, K.; Plotkin, A.; Magee, G.A.; Chung, E.J. miR-145 micelles mitigate atherosclerosis by modulating vascular smooth muscle cell phenotype. Biomaterials 2021, 273, 120810. [Google Scholar] [CrossRef]
- Grewal, N.; Gittenberger-de Groot, A.C.; Poelmann, R.E.; Klautz, R.J.; Lindeman, J.H.; Goumans, M.J.; Palmen, M.; Mohamed, S.A.; Sievers, H.H.; Bogers, A.J.; et al. Ascending aorta dilation in association with bicuspid aortic valve: A maturation defect of the aortic wall. J. Thorac. Cardiovasc. Surg. 2014, 148, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Grewal, N.; Gittenberger-de Groot, A.C. Pathogenesis of aortic wall complications in Marfan syndrome. Cardiovasc. Pathol. 2018, 33, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Grewal, N.; Klautz, R.J.M.; Poelmann, R.E. Intrinsic histological and morphological abnormalities of the pediatric thoracic aorta in bicuspid aortic valve patients are predictive for future aortopathy. Pathol. Res. Pract. 2023, 248, 154620. [Google Scholar] [CrossRef]
- Rabkin, S.W. The Role Matrix Metalloproteinases in the Production of Aortic Aneurysm. Prog. Mol. Biol. Transl. Sci. 2017, 147, 239–265. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Vandooren, J.; Van den Steen, P.E.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): The next decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.M.; Swingler, T.E.; Sampieri, C.L.; Edwards, D.R. The regulation of matrix metalloproteinases and their inhibitors. Int. J. Biochem. Cell Biol. 2008, 40, 1362–1378. [Google Scholar] [CrossRef]
- Bode, W.; Fernandez-Catalan, C.; Tschesche, H.; Grams, F.; Nagase, H.; Maskos, K. Structural properties of matrix metalloproteinases. Cell. Mol. Life Sci. 1999, 55, 639–652. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta 2010, 1803, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, J.S.; Ivey, C.R.; Wheeler, J.B.; Akerman, A.W.; Rice, A.; Patel, R.K.; Stroud, R.E.; Shah, A.A.; Hughes, C.G.; Ferrari, G.; et al. Plasma biomarkers for distinguishing etiologic subtypes of thoracic aortic aneurysm disease. J. Thorac. Cardiovasc. Surg. 2013, 145, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Koullias, G.J.; Ravichandran, P.; Korkolis, D.P.; Rimm, D.L.; Elefteriades, J.A. Increased tissue microarray matrix metalloproteinase expression favors proteolysis in thoracic aortic aneurysms and dissections. Ann. Thorac. Surg. 2004, 78, 2106–2110, discussion 2110–2101. [Google Scholar] [CrossRef]
- Huusko, T.; Salonurmi, T.; Taskinen, P.; Liinamaa, J.; Juvonen, T.; Paakko, P.; Savolainen, M.; Kakko, S. Elevated messenger RNA expression and plasma protein levels of osteopontin and matrix metalloproteinase types 2 and 9 in patients with ascending aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2013, 145, 1117–1123. [Google Scholar] [CrossRef]
- Mi, T.; Nie, B.; Zhang, C.; Zhou, H. The elevated expression of osteopontin and NF-kappaB in human aortic aneurysms and its implication. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 602. [Google Scholar] [CrossRef]
- Ishii, T.; Asuwa, N. Collagen and elastin degradation by matrix metalloproteinases and tissue inhibitors of matrix metalloproteinase in aortic dissection. Hum. Pathol. 2000, 31, 640–646. [Google Scholar] [CrossRef]
- Tscheuschler, A.; Meffert, P.; Beyersdorf, F.; Heilmann, C.; Kocher, N.; Uffelmann, X.; Discher, P.; Siepe, M.; Kari, F.A. MMP-2 Isoforms in Aortic Tissue and Serum of Patients with Ascending Aortic Aneurysms and Aortic Root Aneurysms. PLoS ONE 2016, 11, e0164308. [Google Scholar] [CrossRef]
- Wang, C.; Chang, Q.; Sun, X.; Qian, X.; Liu, P.; Pei, H.; Guo, X.; Liu, W. Angiotensin II Induces an Increase in Matrix Metalloproteinase 2 Expression in Aortic Smooth Muscle Cells of Ascending Thoracic Aortic Aneurysms Through JNK, ERK1/2, and p38 MAPK Activation. J. Cardiovasc. Pharmacol. 2015, 66, 285–293. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Z.; Wu, H.; Yang, Z.; Jiang, W.; Li, L.; Hu, X. Ang II enhances noradrenaline release from sympathetic nerve endings thus contributing to the up-regulation of metalloprotease-2 in aortic dissection patients’ aorta wall. PLoS ONE 2013, 8, e76922. [Google Scholar] [CrossRef]
- Khanafer, K.; Ghosh, A.; Vafai, K. Correlation between MMP and TIMP levels and elastic moduli of ascending thoracic aortic aneurysms. Cardiovasc. Revasc. Med. 2019, 20, 324–327. [Google Scholar] [CrossRef]
- Rabkin, S.W. Differential expression of MMP-2, MMP-9 and TIMP proteins in thoracic aortic aneurysm—Comparison with and without bicuspid aortic valve: A meta-analysis. Vasa 2014, 43, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, A.; Yoshimura, K.; Suzuki, R.; Mikamo, A.; Yamashita, O.; Ikeda, Y.; Tsuchida, M.; Hamano, K. Important role of the angiotensin II pathway in producing matrix metalloproteinase-9 in human thoracic aortic aneurysms. J. Surg. Res. 2013, 183, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Del Porto, F.; di Gioia, C.; Tritapepe, L.; Ferri, L.; Leopizzi, M.; Nofroni, I.; De Santis, V.; Della Rocca, C.; Mitterhofer, A.P.; Bruno, G.; et al. The multitasking role of macrophages in Stanford type A acute aortic dissection. Cardiology 2014, 127, 123–129. [Google Scholar] [CrossRef]
- Song, Y.; Xie, Y.; Liu, F.; Zhao, C.; Yu, R.; Ban, S.; Ye, Q.; Wen, J.; Wan, H.; Li, X.; et al. Expression of matrix metalloproteinase-12 in aortic dissection. BMC Cardiovasc. Disord. 2013, 13, 34. [Google Scholar] [CrossRef]
- Kimura, N.; Futamura, K.; Arakawa, M.; Okada, N.; Emrich, F.; Okamura, H.; Sato, T.; Shudo, Y.; Koyano, T.K.; Yamaguchi, A.; et al. Gene expression profiling of acute type A aortic dissection combined with in vitro assessment. Eur. J. Cardiothorac. Surg. 2017, 52, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Aikawa, M. Many faces of matrix metalloproteinases in aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Vacek, T.P.; Rehman, S.; Neamtu, D.; Yu, S.; Givimani, S.; Tyagi, S.C. Matrix metalloproteinases in atherosclerosis: Role of nitric oxide, hydrogen sulfide, homocysteine, and polymorphisms. Vasc. Health Risk Manag. 2015, 11, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.H.; Cho, C.H.; Kim, H.O.; Jo, Y.H.; Yoon, K.S.; Lee, J.H.; Park, J.C.; Park, K.C.; Ahn, T.B.; Chung, K.C.; et al. Plaque rupture is a determinant of vascular events in carotid artery atherosclerotic disease: Involvement of matrix metalloproteinases 2 and 9. J. Clin. Neurol. 2011, 7, 69–76. [Google Scholar] [CrossRef]
- Rossignol, P.; Ho-Tin-Noe, B.; Vranckx, R.; Bouton, M.C.; Meilhac, O.; Lijnen, H.R.; Guillin, M.C.; Michel, J.B.; Angles-Cano, E. Protease nexin-1 inhibits plasminogen activation-induced apoptosis of adherent cells. J. Biol. Chem. 2004, 279, 10346–10356. [Google Scholar] [CrossRef]
- Kadoglou, N.P.; Liapis, C.D. Matrix metalloproteinases: Contribution to pathogenesis, diagnosis, surveillance and treatment of abdominal aortic aneurysms. Curr. Med. Res. Opin. 2004, 20, 419–432. [Google Scholar] [CrossRef]
- Pyo, R.; Lee, J.K.; Shipley, J.M.; Curci, J.A.; Mao, D.; Ziporin, S.J.; Ennis, T.L.; Shapiro, S.D.; Senior, R.M.; Thompson, R.W. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J. Clin. Investig. 2000, 105, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Longo, G.M.; Xiong, W.; Greiner, T.C.; Zhao, Y.; Fiotti, N.; Baxter, B.T. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J. Clin. Investig. 2002, 110, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Davis, V.; Persidskaia, R.; Baca-Regen, L.; Itoh, Y.; Nagase, H.; Persidsky, Y.; Ghorpade, A.; Baxter, B.T. Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Freestone, T.; Turner, R.J.; Coady, A.; Higman, D.J.; Greenhalgh, R.M.; Powell, J.T. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1145–1151. [Google Scholar] [CrossRef]
- Newman, K.M.; Jean-Claude, J.; Li, H.; Scholes, J.V.; Ogata, Y.; Nagase, H.; Tilson, M.D. Cellular localization of matrix metalloproteinases in the abdominal aortic aneurysm wall. J. Vasc. Surg. 1994, 20, 814–820. [Google Scholar] [CrossRef]
- Newman, K.M.; Malon, A.M.; Shin, R.D.; Scholes, J.V.; Ramey, W.G.; Tilson, M.D. Matrix metalloproteinases in abdominal aortic aneurysm: Characterization, purification, and their possible sources. Connect. Tissue Res. 1994, 30, 265–276. [Google Scholar] [CrossRef]
- Reeps, C.; Pelisek, J.; Seidl, S.; Schuster, T.; Zimmermann, A.; Kuehnl, A.; Eckstein, H.H. Inflammatory infiltrates and neovessels are relevant sources of MMPs in abdominal aortic aneurysm wall. Pathobiology 2009, 76, 243–252. [Google Scholar] [CrossRef]
- Gandhi, R.H.; Irizarry, E.; Cantor, J.O.; Keller, S.; Nackman, G.B.; Halpern, V.J.; Newman, K.M.; Tilson, M.D. Analysis of elastin cross-linking and the connective tissue matrix of abdominal aortic aneurysms. Surgery 1994, 115, 617–620. [Google Scholar]
- Kuzuya, M.; Nakamura, K.; Sasaki, T.; Cheng, X.W.; Itohara, S.; Iguchi, A. Effect of MMP-2 deficiency on atherosclerotic lesion formation in apoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1120–1125. [Google Scholar] [CrossRef]
- Bendeck, M.P.; Zempo, N.; Clowes, A.W.; Galardy, R.E.; Reidy, M.A. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ. Res. 1994, 75, 539–545. [Google Scholar] [CrossRef]
- Zempo, N.; Kenagy, R.D.; Au, Y.P.; Bendeck, M.; Clowes, M.M.; Reidy, M.A.; Clowes, A.W. Matrix metalloproteinases of vascular wall cells are increased in balloon-injured rat carotid artery. J. Vasc. Surg. 1994, 20, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Forough, R.; Koyama, N.; Hasenstab, D.; Lea, H.; Clowes, M.; Nikkari, S.T.; Clowes, A.W. Overexpression of tissue inhibitor of matrix metalloproteinase-1 inhibits vascular smooth muscle cell functions in vitro and in vivo. Circ. Res. 1996, 79, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Southgate, K.M.; Fisher, M.; Banning, A.P.; Thurston, V.J.; Baker, A.H.; Fabunmi, R.P.; Groves, P.H.; Davies, M.; Newby, A.C. Upregulation of basement membrane-degrading metalloproteinase secretion after balloon injury of pig carotid arteries. Circ. Res. 1996, 79, 1177–1187. [Google Scholar] [CrossRef]
- Beck, L., Jr.; D’Amore, P.A. Vascular development: Cellular and molecular regulation. FASEB J. 1997, 11, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Takeda, N.; Hara, H.; Fujiwara, T.; Kanaya, T.; Maemura, S.; Komuro, I. TGF-beta Signaling-Related Genes and Thoracic Aortic Aneurysms and Dissections. Int. J. Mol. Sci. 2018, 19, 2125. [Google Scholar] [CrossRef]
- Cook, J.R.; Clayton, N.P.; Carta, L.; Galatioto, J.; Chiu, E.; Smaldone, S.; Nelson, C.A.; Cheng, S.H.; Wentworth, B.M.; Ramirez, F. Dimorphic effects of transforming growth factor-beta signaling during aortic aneurysm progression in mice suggest a combinatorial therapy for Marfan syndrome. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 911–917. [Google Scholar] [CrossRef]
- Gomez, D.; Al Haj Zen, A.; Borges, L.F.; Philippe, M.; Gutierrez, P.S.; Jondeau, G.; Michel, J.B.; Vranckx, R. Syndromic and non-syndromic aneurysms of the human ascending aorta share activation of the Smad2 pathway. J. Pathol. 2009, 218, 131–142. [Google Scholar] [CrossRef]
- Habashi, J.P.; Judge, D.P.; Holm, T.M.; Cohn, R.D.; Loeys, B.L.; Cooper, T.K.; Myers, L.; Klein, E.C.; Liu, G.; Calvi, C.; et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006, 312, 117–121. [Google Scholar] [CrossRef]
- King, V.L.; Lin, A.Y.; Kristo, F.; Anderson, T.J.; Ahluwalia, N.; Hardy, G.J.; Owens, A.P., 3rd; Howatt, D.A.; Shen, D.; Tager, A.M.; et al. Interferon-gamma and the interferon-inducible chemokine CXCL10 protect against aneurysm formation and rupture. Circulation 2009, 119, 426–435. [Google Scholar] [CrossRef]
- Zilberberg, L.; Phoon, C.K.; Robertson, I.; Dabovic, B.; Ramirez, F.; Rifkin, D.B. Genetic analysis of the contribution of LTBP-3 to thoracic aneurysm in Marfan syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, 14012–14017. [Google Scholar] [CrossRef]
- Chen, X.; Rateri, D.L.; Howatt, D.A.; Balakrishnan, A.; Moorleghen, J.J.; Cassis, L.A.; Daugherty, A. TGF-beta Neutralization Enhances AngII-Induced Aortic Rupture and Aneurysm in Both Thoracic and Abdominal Regions. PLoS ONE 2016, 11, e0153811. [Google Scholar] [CrossRef]
- Wang, Y.; Ait-Oufella, H.; Herbin, O.; Bonnin, P.; Ramkhelawon, B.; Taleb, S.; Huang, J.; Offenstadt, G.; Combadiere, C.; Renia, L.; et al. TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J. Clin. Investig. 2010, 120, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Ferruzzi, J.; Murtada, S.I.; Li, G.; Jiao, Y.; Uman, S.; Ting, M.Y.; Tellides, G.; Humphrey, J.D. Pharmacologically Improved Contractility Protects Against Aortic Dissection in Mice With Disrupted Transforming Growth Factor-beta Signaling Despite Compromised Extracellular Matrix Properties. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Q.; Jiao, Y.; Qin, L.; Ali, R.; Zhou, J.; Ferruzzi, J.; Kim, R.W.; Geirsson, A.; Dietz, H.C.; et al. Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis. J. Clin. Investig. 2014, 124, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.H.; Wei, H.; Jaffe, M.; Airhart, N.; Du, L.; Angelov, S.N.; Yan, J.; Allen, J.K.; Kang, I.; Wight, T.N.; et al. Postnatal Deletion of the Type II Transforming Growth Factor-beta Receptor in Smooth Muscle Cells Causes Severe Aortopathy in Mice. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Hu, J.H.; Angelov, S.N.; Fox, K.; Yan, J.; Enstrom, R.; Smith, A.; Dichek, D.A. Aortopathy in a Mouse Model of Marfan Syndrome Is Not Mediated by Altered Transforming Growth Factor beta Signaling. J. Am. Heart Assoc. 2017, 6, e004968. [Google Scholar] [CrossRef]
- Gadson, P.F., Jr.; Dalton, M.L.; Patterson, E.; Svoboda, D.D.; Hutchinson, L.; Schram, D.; Rosenquist, T.H. Differential response of mesoderm- and neural crest-derived smooth muscle to TGF-beta1: Regulation of c-myb and alpha1 (I) procollagen genes. Exp. Cell Res. 1997, 230, 169–180. [Google Scholar] [CrossRef]
- Thieszen, S.L.; Dalton, M.; Gadson, P.F.; Patterson, E.; Rosenquist, T.H. Embryonic lineage of vascular smooth muscle cells determines responses to collagen matrices and integrin receptor expression. Exp. Cell Res. 1996, 227, 135–145. [Google Scholar] [CrossRef]
- El-Hamamsy, I.; Yacoub, M.H. Cellular and molecular mechanisms of thoracic aortic aneurysms. Nat. Rev. Cardiol. 2009, 6, 771–786. [Google Scholar] [CrossRef]
- Grainger, D.J. Transforming growth factor beta and atherosclerosis: So far, so good for the protective cytokine hypothesis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 399–404. [Google Scholar] [CrossRef]
- McCaffrey, T.A. TGF-betas and TGF-beta receptors in atherosclerosis. Cytokine Growth Factor. Rev. 2000, 11, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Neptune, E.R.; Frischmeyer, P.A.; Arking, D.E.; Myers, L.; Bunton, T.E.; Gayraud, B.; Ramirez, F.; Sakai, L.Y.; Dietz, H.C. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003, 33, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Nataatmadja, M.; West, J.; West, M. Overexpression of transforming growth factor-beta is associated with increased hyaluronan content and impairment of repair in Marfan syndrome aortic aneurysm. Circulation 2006, 114, I371–I377. [Google Scholar] [CrossRef] [PubMed]
- Lutgens, E.; Daemen, M.J. Transforming growth factor-beta: A local or systemic mediator of plaque stability? Circ. Res. 2001, 89, 853–855. [Google Scholar] [CrossRef]
- Mallat, Z.; Gojova, A.; Marchiol-Fournigault, C.; Esposito, B.; Kamate, C.; Merval, R.; Fradelizi, D.; Tedgui, A. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ. Res. 2001, 89, 930–934. [Google Scholar] [CrossRef]
- McCaffrey, T.A.; Consigli, S.; Du, B.; Falcone, D.J.; Sanborn, T.A.; Spokojny, A.M.; Bush, H.L., Jr. Decreased type II/type I TGF-beta receptor ratio in cells derived from human atherosclerotic lesions. Conversion from an antiproliferative to profibrotic response to TGF-beta1. J. Clin. Investig. 1995, 96, 2667–2675. [Google Scholar] [CrossRef]
- Andreotti, F.; Porto, I.; Crea, F.; Maseri, A. Inflammatory gene polymorphisms and ischaemic heart disease: Review of population association studies. Heart 2002, 87, 107–112. [Google Scholar] [CrossRef]
- Humphries, S.E.; Luong, L.A.; Talmud, P.J.; Frick, M.H.; Kesaniemi, Y.A.; Pasternack, A.; Taskinen, M.R.; Syvanne, M. The 5A/6A polymorphism in the promoter of the stromelysin-1 (MMP-3) gene predicts progression of angiographically determined coronary artery disease in men in the LOCAT gemfibrozil study. Lopid Coronary Angiography Trial. Atherosclerosis 1998, 139, 49–56. [Google Scholar] [CrossRef]
- Kempf, K.; Haltern, G.; Futh, R.; Herder, C.; Muller-Scholze, S.; Gulker, H.; Martin, S. Increased TNF-alpha and decreased TGF-beta expression in peripheral blood leukocytes after acute myocardial infarction. Horm. Metab. Res. 2006, 38, 346–351. [Google Scholar] [CrossRef]
- Koch, W.; Hoppmann, P.; Mueller, J.C.; Schomig, A.; Kastrati, A. Association of transforming growth factor-beta1 gene polymorphisms with myocardial infarction in patients with angiographically proven coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1114–1119. [Google Scholar] [CrossRef]
- Vaughan, C.J.; Casey, M.; He, J.; Veugelers, M.; Henderson, K.; Guo, D.; Campagna, R.; Roman, M.J.; Milewicz, D.M.; Devereux, R.B.; et al. Identification of a chromosome 11q23.2-q24 locus for familial aortic aneurysm disease, a genetically heterogeneous disorder. Circulation 2001, 103, 2469–2475. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Eriksson, P.; Hamsten, A.; Kurkinen, M.; Humphries, S.E.; Henney, A.M. Progression of coronary atherosclerosis is associated with a common genetic variant of the human stromelysin-1 promoter which results in reduced gene expression. J. Biol. Chem. 1996, 271, 13055–13060. [Google Scholar] [CrossRef] [PubMed]
- Yokota, M.; Ichihara, S.; Lin, T.L.; Nakashima, N.; Yamada, Y. Association of a T29-->C polymorphism of the transforming growth factor-beta1 gene with genetic susceptibility to myocardial infarction in Japanese. Circulation 2000, 101, 2783–2787. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Al-Sammarraie, N.; Gebere, M.G.; Bhattacharya, A.; Chopra, S.; Johnson, J.; Pena, E.A.; Eberth, J.F.; Poelmann, R.E.; Gittenberger-de Groot, A.C.; et al. Transforming Growth Factor Beta3 is Required for Cardiovascular Development. J. Cardiovasc. Dev. Dis. 2020, 7, 19. [Google Scholar] [CrossRef]
- Grewal, N.; Girdauskas, E.; Idhrees, M.; Velayudhan, B.; Klautz, R.; Driessen, A.; Poelmann, R.E. Structural abnormalities in the non-dilated ascending aortic wall of bicuspid aortic valve patients. Cardiovasc. Pathol. 2023, 62, 107478. [Google Scholar] [CrossRef]
- Moncada, S. Adventures in vascular biology: A tale of two mediators. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 735–759. [Google Scholar] [CrossRef]
- Griffith, T.M. Endothelial control of vascular tone by nitric oxide and gap junctions: A haemodynamic perspective. Biorheology 2002, 39, 307–318. [Google Scholar]
- Pohl, U.; Holtz, J.; Busse, R.; Bassenge, E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension 1986, 8, 37–44. [Google Scholar] [CrossRef]
- Davies, P.F. Flow-mediated endothelial mechanotransduction. Physiol. Rev. 1995, 75, 519–560. [Google Scholar] [CrossRef]
- Garcia-Cardena, G.; Comander, J.I.; Blackman, B.R.; Anderson, K.R.; Gimbrone, M.A. Mechanosensitive endothelial gene expression profiles: Scripts for the role of hemodynamics in atherogenesis? Ann. N. Y. Acad. Sci. 2001, 947, 1–6. [Google Scholar] [CrossRef]
- Suo, J.; Oshinski, J.N.; Giddens, D.P. Blood flow patterns in the proximal human coronary arteries: Relationship to atherosclerotic plaque occurrence. Mol. Cell. Biomech. 2008, 5, 9–18. [Google Scholar] [PubMed]
- Davies, P.F.; Polacek, D.C.; Handen, J.S.; Helmke, B.P.; DePaola, N. A spatial approach to transcriptional profiling: Mechanotransduction and the focal origin of atherosclerosis. Trends Biotechnol. 1999, 17, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Steinman, D.A.; Taylor, C.A. Flow imaging and computing: Large artery hemodynamics. Ann. Biomed. Eng. 2005, 33, 1704–1709. [Google Scholar] [CrossRef]
- Hajra, L.; Evans, A.I.; Chen, M.; Hyduk, S.J.; Collins, T.; Cybulsky, M.I. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc. Natl. Acad. Sci. USA 2000, 97, 9052–9057. [Google Scholar] [CrossRef] [PubMed]
- Passerini, A.G.; Polacek, D.C.; Shi, C.; Francesco, N.M.; Manduchi, E.; Grant, G.R.; Pritchard, W.F.; Powell, S.; Chang, G.Y.; Stoeckert, C.J., Jr.; et al. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc. Natl. Acad. Sci. USA 2004, 101, 2482–2487. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Kim, S.H. Pulse Wave Velocity in Atherosclerosis. Front. Cardiovasc. Med. 2019, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Witteman, J.C.; Grobbee, D.E.; Valkenburg, H.A.; van Hemert, A.M.; Stijnen, T.; Burger, H.; Hofman, A. J-shaped relation between change in diastolic blood pressure and progression of aortic atherosclerosis. Lancet 1994, 343, 504–507. [Google Scholar] [CrossRef]
- Dao, H.H.; Essalihi, R.; Bouvet, C.; Moreau, P. Evolution and modulation of age-related medial elastocalcinosis: Impact on large artery stiffness and isolated systolic hypertension. Cardiovasc. Res. 2005, 66, 307–317. [Google Scholar] [CrossRef]
- Ohyama, Y.; Ambale-Venkatesh, B.; Noda, C.; Kim, J.Y.; Tanami, Y.; Teixido-Tura, G.; Chugh, A.R.; Redheuil, A.; Liu, C.Y.; Wu, C.O.; et al. Aortic Arch Pulse Wave Velocity Assessed by Magnetic Resonance Imaging as a Predictor of Incident Cardiovascular Events: The MESA (Multi-Ethnic Study of Atherosclerosis). Hypertension 2017, 70, 524–530. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waldron, C.; Zafar, M.A.; Ziganshin, B.A.; Weininger, G.; Grewal, N.; Elefteriades, J.A. Evidence Accumulates: Patients with Ascending Aneurysms Are Strongly Protected from Atherosclerotic Disease. Int. J. Mol. Sci. 2023, 24, 15640. https://doi.org/10.3390/ijms242115640
Waldron C, Zafar MA, Ziganshin BA, Weininger G, Grewal N, Elefteriades JA. Evidence Accumulates: Patients with Ascending Aneurysms Are Strongly Protected from Atherosclerotic Disease. International Journal of Molecular Sciences. 2023; 24(21):15640. https://doi.org/10.3390/ijms242115640
Chicago/Turabian StyleWaldron, Christina, Mohammad A. Zafar, Bulat A. Ziganshin, Gabe Weininger, Nimrat Grewal, and John A. Elefteriades. 2023. "Evidence Accumulates: Patients with Ascending Aneurysms Are Strongly Protected from Atherosclerotic Disease" International Journal of Molecular Sciences 24, no. 21: 15640. https://doi.org/10.3390/ijms242115640
APA StyleWaldron, C., Zafar, M. A., Ziganshin, B. A., Weininger, G., Grewal, N., & Elefteriades, J. A. (2023). Evidence Accumulates: Patients with Ascending Aneurysms Are Strongly Protected from Atherosclerotic Disease. International Journal of Molecular Sciences, 24(21), 15640. https://doi.org/10.3390/ijms242115640