Lithium Treatment Induces Cardiac Dysfunction in Mice
Abstract
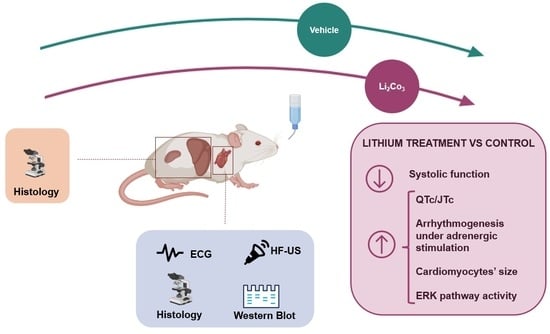
:1. Introduction
2. Results
2.1. Animal Well-Being and Plasma Li Levels
2.2. Li Administration Impairs Both Systolic Function and Ventricular Repolarisation
2.3. Effect of Li Intake on the Arrhythmogenic Response to β-Adrenergic Stimulation
2.4. The Effect of Li Treatment on Cardiomyocyte Morphometry and Cardiac Tissue
2.5. Li Upregulates Hypertrophic Signalling
2.6. The Histopathological Effect of Li on the Liver and Kidney
3. Discussion and Conclusions
Conclusions
4. Materials and Methods
4.1. Animals and Experimental Protocol
4.2. Li Administration
4.3. High-Frequency Ultrasound Examinations
4.4. Electrocardiographic Recordings and Analysis
4.5. Arrhythmogenic Response to β-Adrenergic Challenge
4.6. Plasma Determination of Li
4.7. Histological Analysis Studies
4.8. Protein Extraction and Western Blotting Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wietelmann, U.; Klet, J. 200 years of Lithium and 100 years of organolithium chemistry. Z. Anorg. Allg. Chem. 2018, 644, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Aral, H.; Vecchio-Sadus, A. Toxicity of lithium to humans and the environment—A literature review. Ecotoxicol. Environ. Saf. 2008, 70, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Ammari, T.G.; Al-Zu’bi, Y.; Abu-Baker, S.; Dababneh, B.; Gnemat, W.; Tahboub, A. The occurrence of lithium in the environment of the Jordan Valley and its transfer into the food chain. Environ. Geochem. Health 2011, 33, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, B.; Tanveer, M.; Hassan, W.; Shah, A.N.; Anjum, S.A.; Cheema, S.A.; Ali, I. Lithium toxicity in plants: Reasons, mechanisms and remediation possibilities—A review. Plant Physiol. Biochem. 2016, 107, 104–115. [Google Scholar] [CrossRef]
- Shakoor, N.; Adeel, M.; Ahmad, M.A.; Zain, M.; Waheed, U.; Javaid, R.A.; Haider, F.U.; Azeem, I.; Zhou, P.; Li, Y.; et al. Reimagining safe lithium applications in the living environment and its impacts on human, animal, and plant system. Environ. Sci. Ecotechnol. 2023, 15, 100252. [Google Scholar] [CrossRef]
- Volkmann, C.; Bschor, T.; Köhler, S. Lithium treatment over the lifespan in bipolar disorders. Front. Psychiatry 2020, 11, 377. [Google Scholar] [CrossRef]
- Tondo, L.; Alda, M.; Bauer, M.; Bergink, V.; Grof, P.; Hajek, T.; Lewitka, U.; Licht, R.W.; Manchia, M.; Müller-Oerlinghausen, B.; et al. International Group for Studies of Lithium (IGSLi). Clinical use of lithium salts: Guide for users and prescribers. Int. J. Bipolar Disord. 2019, 7, 16. [Google Scholar] [CrossRef]
- Matsunaga, S.; Kishi, T.; Annas, P.; Basun, H.; Hampel, H.; Iwata, N. Lithium as a treatment for Alzheimer’s Disease: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2015, 48, 403–410. [Google Scholar] [CrossRef]
- Scherf-Clavel, M.; Treiber, D.S.; Deckert, J.; Unterecker, S.; Hommers, L. Drug-drug interactions between lithium and cardiovascular as well as anti-inflammatory drugs. Pharmacopsychiatry 2020, 53, 229–234. [Google Scholar] [CrossRef]
- Dunner, D.L. Optimizing lithium treatment. J. Clin. Psychiatry. 2000, 1 (Suppl. S9), 76–81. [Google Scholar]
- Öhlund, L.; Ott, M.; Oja, S.; Bergqvist, M.; Lundqvist, R.; Sandlund, M.; Salander Renberg, E.; Werneke, U. Reasons for lithium discontinuation in men and women with bipolar disorder: A retrospective cohort study. BMC Psychiatry 2018, 18, 37, Erratum in BMC Psychiatry 2018, 18, 322. [Google Scholar] [CrossRef] [PubMed]
- Gitlin, M. Lithium side effects and toxicity: Prevalence and management strategies. Int. J. Bipolar Disord. 2016, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Murru, A.; Popovic, D.; Pacchiarotti, I.; Hidalgo, D.; León-Caballero, J.; Vieta, E. Management of adverse effects of mood stabilizers. Curr. Psychiatry Rep. 2015, 17, 603. [Google Scholar] [CrossRef] [PubMed]
- Ferensztajn-Rochowiak, E.; Rybakowski, J.K. Long-term lithium therapy: Side effects and interactions. Pharmaceuticals 2023, 16, 74. [Google Scholar] [CrossRef]
- Khalid, M.; Sheikh, W.; Sherazi, M.; Imran, T.F. Lithium-induced bradycardia and cardiomyopathy in a patient with bipolar disorder and paranoid schizophrenia. Cureus 2023, 15, e40949. [Google Scholar] [CrossRef]
- Corriveau, S.; Gardhouse, A.; Soth, M.; Ainsworth, C. Lithium Toxicity. Can. J. Gen. Intern. Med. 2013, 8, 69–71. [Google Scholar] [CrossRef]
- Mehta, N.; Vannozzi, R. Lithium-induced electrocardiographic changes: A complete review. Clin. Cardiol. 2017, 40, 1363–1367. [Google Scholar] [CrossRef]
- Diserens, L.; Porretta, A.P.; Trana, C.; Meier, D. Lithium-induced ECG modifications: Navigating from acute coronary syndrome to Brugada syndrome. BMJ Case Rep. 2021, 14, e241555. [Google Scholar] [CrossRef]
- Sarangi, A.; Javed, S.; Paul, T.; Amor, W. Lithium-induced sinoatrial node dysfunction. Cureus 2021, 13, e16778. [Google Scholar] [CrossRef]
- Varga, Z.V.; Ferdinandy, P.; Liaudet, L.; Pacher, P. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1453–H1467. [Google Scholar] [CrossRef]
- Vodovar, D.; El Balkhi, S.; Curis, E.; Deye, N.; Mégarbane, B. Lithium poisoning in the intensive care unit: Predictive factors of severity and indications for extracorporeal toxin removal to improve outcome. Clin. Toxicol. 2016, 54, 615–623. [Google Scholar] [CrossRef]
- Truedson, P.; Ott, M.; Lindmark, K.; Ström, M.; Maripuu, M.; Lundqvist, R.; Werneke, U. Effects of toxic lithium levels on ECG-findings from the LiSIE retrospective cohort study. J. Clin. Med. 2022, 11, 5941. [Google Scholar] [CrossRef] [PubMed]
- Mezni, A.; Aoua, H.; Khazri, O.; Limam, F.; Aouani, E. Lithium induced oxidative damage and inflammation in the rat’s heart: Protective effect of grape seed and skin extract. Biomed. Pharmacother. 2017, 95, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Gholamifar, E.; Naserzadeh, P.; Hosseini, M.J.; Pourahmad, J. Toxicity of lithium on isolated heart mitochondria and cardiomyocyte: A justification for its cardiotoxic adverse effect. J. Biochem. Mol. Toxicol. 2017, 31, e21836. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.A.; Bhat, G.M.; Shadad, S.; Lone, M.M. Lithium carbonate induced histopathological changes in the heart of albino rats. World J. Pharm. Pharm. Sci. 2015, 4, 1684–1692. [Google Scholar]
- Ben Saad, A.; Rjeibi, I.; Alimi, H.; Ncib, S.; Smida, A.; Zouari, N.; Zourgui, L. Lithium induced, oxidative stress and related damages in testes and heart in male rats: The protective effects of Malva sylvestris extract. Biomed. Pharmacother. 2017, 86, 127–135. [Google Scholar] [CrossRef]
- Abdel Hamid, O.I.; Ibrahim, E.M.; Hussein, M.H.; Elkhateeb, S.A. The molecular mechanisms of lithium-induced cardiotoxicity in male rats and its amelioration by N-acetyl cysteine. Hum. Exp. Toxicol. 2020, 39, 696–711. [Google Scholar] [CrossRef]
- Ahmad, M.; Elnakady, Y.; Farooq, M.; Wadaan, M. Lithium induced toxicity in rats: Blood serum chemistry, antioxidative enzymes in red blood cells and histopathological studies. Biol. Pharm. Bull. 2011, 34, 272–277. [Google Scholar] [CrossRef]
- Ben Saad, A.; Rjeibi, I.; Brahmi, D.; Smida, A.; Ncib, S.; Zouari, N.; Zourgui, L. Malva sylvestris extract protects upon lithium carbonate-induced kidney damages in male rat. Biomed. Pharmacother. 2016, 84, 1099–1107. [Google Scholar] [CrossRef]
- Ben Saad, A.; Dalel, B.; Rjeibi, I.; Smida, A.; Ncib, S.; Zouari, N.; Zourgui, L. Phytochemical, antioxidant and protective effect of cactus cladodes extract against lithium-induced liver injury in rats. Pharm. Biol. 2017, 55, 516–525. [Google Scholar] [CrossRef]
- Mozos, I.; Filimon, L. QT and Tpeak-Tend intervals in shift workers. J Electrocardiol. 2013, 46, 60–65. [Google Scholar] [CrossRef]
- Reilly, J.G.; Ayis, S.A.; Ferrier, I.N.; Jones, S.J.; Thomas, S.H.L. QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet 2000, 355, 1048–1052. [Google Scholar] [CrossRef]
- Mamiya, K.; Sadanaga, T.; Sekita, A.; Nabeyama, Y.; Yao, H.; Yukawa, E. Lithium concentration correlates with QTc in patients with psychosis. J. Electrocardiol. 2005, 38, 148–151. [Google Scholar] [CrossRef]
- Chen, P.H.; Kao, H.Y.H.; Chang, C.K.; Lin, Y.K.; Lin, Y.F.; Chen, Y.Y. Clinical risk factors for therapeutic Lithium-associated electrocardiographic changes in patients with bipolar disorder. J. Clin. Psychopharmacol. 2021, 40, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Chen, Y.-C.; Lu, Y.-Y.; Lin, Y.-K.; Higa, S.; Chen, S.-A.; Chen, Y.-J. Gender difference in Lithium-induced sodium current dysregulation and ventricular arrhythmogenesis in right ventricular outflow tract cardiomyocytes. Biomedicines 2022, 10, 2727. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Lu, Y.Y.; Chen, Y.C.; Chang, C.J.; Kao, Y.H.; Lin, Y.K.; Chen, Y.H.; Chen, S.A.; Yang, L.Y.; Chen, Y.J. Ablation of androgenreceptor gene triggers right ventricular outflow tract ventricular tachycardia. Int. J. Cardiol. 2015, 189, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Vavrenyuk, A.; Estep, Z.; Bahgat, J.; Jasty, R.; Miller, W.; His, D. Long-term lithium carbonate use and hypertrophic cardiomyopathy. Is there a possible link? JACC 2021, 77 (Suppl. S1), 2242. [Google Scholar] [CrossRef]
- McMullen, J.R.; Shioi, T.; Zhang, L.; Tarnavski, O.; Sherwood, M.C.; Kang, P.M.; Izumo, S. Phosphoinositide 3-kinase(p110α) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 2003, 100, 12355–12360. [Google Scholar] [CrossRef]
- Lee, T.M.; Lin, S.Z.; Chang, N.C. Effect of lithium on ventricular remodelling in infarcted rats via the Akt/mTOR signalling pathways. Biosci. Rep. 2017, 37, BSR20160257. [Google Scholar] [CrossRef]
- Hu, B.; Song, J.T.; Ji, X.F.; Liu, Z.Q.; Cong, M.L.; Liu, D.X. Sodium Ferulate protects against angiotensin II-Induced cardiac hypertrophy in mice by regulating the MAPK/ERK and JNK pathways. Biomed. Res. Int. 2017, 2017, 3754942. [Google Scholar] [CrossRef]
- Tamura, S.; Marunouchi, T.; Tanonaka, K. Heat-shock protein 90 modulates cardiac ventricular hypertrophy via activation of MAPK pathway. J. Mol. Cell Cardiol. 2019, 127, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.P.; Li, J.T.; Zeng, N.; Ni, G.X. Role of extracellular signal-regulated kinase 1/2 signaling underlying cardiac hypertrophy. Cardiol. J. 2021, 28, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhang, N.; Liao, H.; Chen, S.; Xu, L.; Li, J.; Yang, Z.; Deng, W.; Tang, Q. Caffeic acid phenethyl ester attenuates pathological cardiac hypertrophy by regulation of MEK/ERK signalling pathway in vivo and vitro. Life Sci. 2017, 181, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Saleem, N.; Prasad, A.; Goswami, S.K. Apocynin prevents isoproterenol-induced cardiac hypertrophy in rat. Mol. Cell Biochem. 2018, 445, 79–88. [Google Scholar] [CrossRef]
- Mao, Q.; Wu, S.; Peng, C.; Peng, B.; Luo, X.; Huang, L.; Zhang, H. Interactions between the ERK1/2 signaling pathway and PCAF play a key role in PE-induced cardiomyocyte hypertrophy. Mol. Med. Rep. 2021, 24, 636. [Google Scholar] [CrossRef] [PubMed]
- Hardt, S.E.; Tomita, H.; Katus, H.A.; Sadoshima, J. Phosphorylation of eukaryotic translation initiation factor 2Bepsilon by glycogen synthase kinase-3beta regulates beta-adrenergic cardiac myocyte hypertrophy. Circ. Res. 2004, 94, 926–935. [Google Scholar] [CrossRef]
- Tateishi, A.; Matsushita, M.; Asai, T.; Masuda, Z.; Kuriyama, M.; Kanki, K.; Ishino, K.; Kawada, M.; Sano, S.; Matsui, H. Effect of inhibition of glycogen synthase kinase-3 on cardiac hypertrophy during acute pressure overload. Gen. Thorac. Cardiovasc. Surg. 2010, 58, 265–270. [Google Scholar] [CrossRef]
- Klein, P.S.; Melton, D.A. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA. 1996, 93, 8455–8459. [Google Scholar] [CrossRef]
- Stambolic, V.; Ruel, L.; Woodgett, J.R. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 1996, 6, 1664–1668. [Google Scholar] [CrossRef]
- O’Brien, W.T.; Klein, P.S. Validating GSK3 as an in vivo target of lithium action. Biochem. Soc. Trans. 2009, 37, 1133–1138. [Google Scholar] [CrossRef]
- Freland, L.; Beaulieu, J.-M. Inhibition of GSK3 by lithium, from single molecules to signaling networks. Front. Mol. Neurosci. 2012, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, E.; Argüello-Miranda, O.; Chiu, S.W.; Fazal, Z.; Kruczek, J.; Nunez-Corrales, S.; Pandit, S.; Pritchet, L. Towards a unified understanding of lithium action in basic biology and its significance for applied biology. J. Membr. Biol. 2017, 250, 587–604. [Google Scholar] [CrossRef] [PubMed]
- Flepisi, T.B.; Lochner, A.; Huisamen, B. The consequences of long-term glycogen synthase kinase-3 inhibition on normal and insulin resistant rat hearts. Cardiovasc. Drugs Ther. 2013, 27, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef]
- Hamstra, S.I.; Kurgan, N.; Baranowski, R.W.; Qiu, L.; Watson, C.J.F.; Messner, H.N.; MacPherson, R.E.K.; MacNeil, A.J.; Roy, B.D.; Fajardo, V.A. Low-dose lithium feeding increases the SERCA2a-to-phospholamban ratio, improving SERCA function in murine left ventricles. Exp. Physiol. 2020, 105, 666–675. [Google Scholar] [CrossRef]
- Shen, J.; Li, X.; Shi, X.; Wang, W.; Zhou, H.; Wu, J.; Wang, X.; Li, J. The toxicity of lithium to human cardiomyocytes. Environ. Sci. Eur. 2020, 32, 59. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and humans. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Mitchell, G.F.; Jeron, A.; Koren, G. Measurement of heart rate and Q-T interval in the conscious mouse. Am. J. Physiol. 1998, 274, H747–H751. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
L’Abbate, S.; Nicolini, G.; Marchetti, S.; Forte, G.; Lepore, E.; Unfer, V.; Kusmic, C. Lithium Treatment Induces Cardiac Dysfunction in Mice. Int. J. Mol. Sci. 2023, 24, 15872. https://doi.org/10.3390/ijms242115872
L’Abbate S, Nicolini G, Marchetti S, Forte G, Lepore E, Unfer V, Kusmic C. Lithium Treatment Induces Cardiac Dysfunction in Mice. International Journal of Molecular Sciences. 2023; 24(21):15872. https://doi.org/10.3390/ijms242115872
Chicago/Turabian StyleL’Abbate, Serena, Giuseppina Nicolini, Sabrina Marchetti, Gianpiero Forte, Elisa Lepore, Virginia Unfer, and Claudia Kusmic. 2023. "Lithium Treatment Induces Cardiac Dysfunction in Mice" International Journal of Molecular Sciences 24, no. 21: 15872. https://doi.org/10.3390/ijms242115872
APA StyleL’Abbate, S., Nicolini, G., Marchetti, S., Forte, G., Lepore, E., Unfer, V., & Kusmic, C. (2023). Lithium Treatment Induces Cardiac Dysfunction in Mice. International Journal of Molecular Sciences, 24(21), 15872. https://doi.org/10.3390/ijms242115872