Evidence-Based Approach for Secondary Prevention of Uterine Fibroids (The ESCAPE Approach)
Abstract
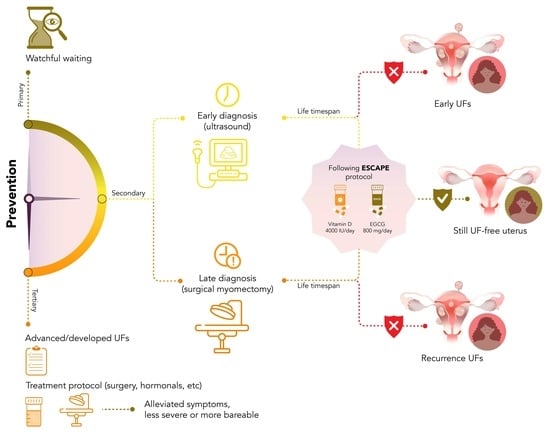
:1. Introduction
2. Vitamin D Serum Level Checkup and Proper Supplementation
3. Epigallocatechin Gallate (EGCG), and Green Tea Extract Consumption
4. Summary of Supporting Clinical Trials
5. Avoiding Phthalate Exposure
- Use phthalate-free water bottles.
- Stay hydrated with filtered water.
- Include detox foods in the diet.
- Adopt a plant-based diet intake.
- Remove shoes before entering the home.
- Avoid microwaving plastics.
- Reduce the use of beauty products.
- Avoid nail polish.
- Minimize pesticide exposure.
- Use trappers without plastics at home.
- Wash hands regularly.
- Dust and vacuum often with a HEPA-filtered vacuum.
- Choose fresh whole foods over processed ones.
- Avoid air fresheners.
- Remove the top layer of food before usage.
- Limit shellfish consumption.
- Reduce the use of plastic containers.
6. Anti-UF Diet
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borah, B.J.; Nicholson, W.K.; Bradley, L.; Stewart, E.A. The impact of uterine leiomyomas: A national survey of affected women. Am. J. Obstet. Gynecol. 2013, 209, 319.e1–319.e20. [Google Scholar] [CrossRef] [PubMed]
- Sefah, N.; Ndebele, S.; Prince, L.; Korasare, E.; Agbleke, M.; Nkansah, A.; Thompson, H.; Al-Hendy, A.; Agbleke, A.A. Uterine fibroids—Causes, impact, treatment, and lens to the African perspective. Front. Pharmacol. 2022, 13, 1045783. [Google Scholar] [CrossRef] [PubMed]
- Anneveldt, K.J.; Nijholt, I.M.; Schutte, J.M.; Dijkstra, J.R.; Frederix, G.W.J.; Ista, E.; Verpalen, I.M.; Veersema, S.; Huirne, J.A.F.; Hehenkamp, W.J.K.; et al. Comparison of (Cost-)Effectiveness of Magnetic Resonance Image-Guided High-Intensity-Focused Ultrasound with Standard (Minimally) Invasive Fibroid Treatments: Protocol for a Multicenter Randomized Controlled Trial (MYCHOICE). JMIR Res. Protoc. 2021, 10, e29467. [Google Scholar] [CrossRef]
- Giuliani, E.; As-Sanie, S.; Marsh, E.E. Epidemiology and management of uterine fibroids. Int. J. Gynaecol. Obstet. 2020, 149, 3–9. [Google Scholar] [CrossRef]
- Cheng, L.-C.; Li, H.-Y.; Gong, Q.-Q.; Huang, C.-Y.; Zhang, C.; Yan, J.-Z. Global, regional, and national burden of uterine fibroids in the last 30 years: Estimates from the 1990 to 2019 Global Burden of Disease Study. Front. Med. 2022, 9, 1003605. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Swetha, P.; Nayak, S.; Singh, S. The Association Between Ferritin and Vitamin D Levels in Premenopausal Fibroid Uterus Cases with Anemia. Cureus 2021, 13, e13392. [Google Scholar] [CrossRef]
- Anonymous. Management of Symptomatic Uterine Leiomyomas: ACOG Practice Bulletin, Number 228. Obstet. Gynecol. 2021, 137, e100–e115. [Google Scholar] [CrossRef]
- Soliman, A.M.; Margolis, M.K.; Castelli-Haley, J.; Fuldeore, M.J.; Owens, C.D.; Coyne, K.S. Impact of uterine fibroid symptoms on health-related quality of life of US women: Evidence from a cross-sectional survey. Curr. Med. Res. Opin. 2017, 33, 1971–1978. [Google Scholar] [CrossRef]
- Al-Hendy, A.; Myers, E.R.; Stewart, E. Uterine Fibroids: Burden and Unmet Medical Need. Semin. Reprod. Med. 2017, 35, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Elkafas, H.; Badary, O.A.; Elmorsy, E.; Kamel, R.; Yang, Q.; Al-Hendy, A. Endocrine-Disrupting Chemicals and Vitamin D Deficiency in the Pathogenesis of Uterine Fibroids. J. Adv. Pharm. Res. 2021, 5, 260–275. [Google Scholar] [CrossRef]
- Salehi, A.M.; Jenabi, E.; Farashi, S.; Aghababaei, S.; Salimi, Z. The environmental risk factors related to uterine leiomyoma: An umbrella review. J. Gynecol. Obstet. Hum. Reprod. 2023, 52, 102517. [Google Scholar] [CrossRef]
- Yang, Q.; Ciebiera, M.; Bariani, M.V.; Ali, M.; Elkafas, H.; Boyer, T.G.; Al-Hendy, A. Comprehensive Review of Uterine Fibroids: Developmental Origin, Pathogenesis, and Treatment. Endocr. Rev. 2021, 43, 678–719. [Google Scholar] [CrossRef]
- He, Y.; Zeng, Q.; Dong, S.; Qin, L.; Li, G.; Wang, P. Associations between uterine fibroids and lifestyles including diet, physical activity and stress: A case-control study in China. Asia Pac. J. Clin. Nutr. 2013, 22, 109–117. [Google Scholar]
- Keaton, J.M.; Jasper, E.A.; Hellwege, J.N.; Jones, S.H.; Torstenson, E.S.; Edwards, T.L.; Edwards, D.R.V. Evidence that geographic variation in genetic ancestry associates with uterine fibroids. Hum. Genet. 2021, 140, 1433–1440. [Google Scholar] [CrossRef]
- Stewart, E.; Cookson, C.; Gandolfo, R.; Schulze-Rath, R. Epidemiology of uterine fibroids: A systematic review. BJOG 2017, 124, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Kisling, L.A.; Das, M.J. Prevention Strategies. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537222/ (accessed on 22 September 2023).
- Ali, M.; Bariani, M.V.; Vafaei, S.; Omran, M.M.; Yang, Q.; Madueke-Laveaux, O.S.; Al-Hendy, A. Prevention of uterine fibroids: Molecular mechanisms and potential clinical application. J. Endometr. Uterine Disord. 2023, 1, 100018. [Google Scholar] [CrossRef]
- De La Cruz, M.S.D.; Buchanan, E.M. Uterine Fibroids: Diagnosis and Treatment. Am. Fam. Physician 2017, 95, 100–107. [Google Scholar] [PubMed]
- Baird, D.D.; Harmon, Q.E.; Upson, K.; Moore, K.R.; Barker-Cummings, C.; Baker, S.; Cooper, T.; Wegienka, G. A Prospective, Ultrasound-Based Study to Evaluate Risk Factors for Uterine Fibroid Incidence and Growth: Methods and Results of Recruitment. J. Womens Health 2015, 24, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.; Kurzawa, P.; Bednarek, W.; Gryboś, A.; Mardas, M.; Krzyżaniak, M.; Majewski, J.; Markowska, J.; Gryboś, M.; Żurawski, J. Immunohistochemical Expression of Vitamin D Receptor in Uterine Fibroids. Nutrients 2022, 14, 3371. [Google Scholar] [CrossRef]
- Catherino, W.H.; Eltoukhi, H.M.; Al-Hendy, A. Racial and ethnic differences in the pathogenesis and clinical manifestations of uterine leiomyoma. Semin. Reprod. Med. 2013, 31, 370–379. [Google Scholar] [CrossRef]
- Sharan, C.; Halder, S.K.; Thota, C.; Jaleel, T.; Nair, S.; Al-Hendy, A. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-O-methyltransferase. Fertil. Steril. 2011, 95, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Jayes, F.L.; Liu, B.; Moutos, F.T.; Kuchibhatla, M.; Guilak, F.; Leppert, P.C. Loss of stiffness in collagen-rich uterine fibroids after digestion with purified collagenase Clostridium histolyticum. Am. J. Obstet. Gynecol. 2016, 215, 596.e1–596.e8. [Google Scholar] [CrossRef]
- Roshdy, E.; Rajaratnam, V.; Maitra, S.; Sabry, M.; Allah, A.S.; Al-Hendy, A. Treatment of symptomatic uterine fibroids with green tea extract: A pilot randomized controlled clinical study. Int. J. Womens Health 2013, 5, 477–486. [Google Scholar]
- Brunengraber, L.N.; Jayes, F.L.; Leppert, P.C. Injectable Clostridium histolyticum collagenase as a potential treatment for uterine fibroids. Reprod. Sci. 2014, 21, 1452–1459. [Google Scholar] [CrossRef]
- Kashani, B.N.; Centini, G.; Morelli, S.S.; Weiss, G.; Petraglia, F. Role of Medical Management for Uterine Leiomyomas. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 34, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Madueke-Laveaux, O.S.; Ciebiera, M.; Al-Hendy, A. GnRH analogs for the treatment of heavy menstrual bleeding associated with uterine fibroids. F&S Rep. 2023, 4 (Suppl. S2), 46–50. [Google Scholar]
- Ciebiera, M.; Madueke-Laveaux, O.S.; Feduniw, S.; Ulin, M.; Spaczynski, R.; Zgliczyńska, M.; Bączkowska, M.; Zarychta, E.; Łoziński, T.; Ali, M.; et al. GnRH agonists and antagonists in therapy of symptomatic uterine fibroids—Current roles and future perspectives. Expert. Opin. Pharmacother 2023, 24, 1799–1809. [Google Scholar] [CrossRef]
- Sohn, G.S.; Cho, S.; Kim, Y.M.; Cho, C.-H.; Kim, M.-R.; Lee, S.R. Current medical treatment of uterine fibroids. Obstet. Gynecol. Sci. 2018, 61, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Raslan, M.; Ciebiera, M.; Zaręba, K.; Al-Hendy, A. Current approaches to overcome the side effects of GnRH analogs in the treatment of patients with uterine fibroids. Expert. Opin. Drug Saf. 2022, 21, 477–486. [Google Scholar] [CrossRef]
- Ali, M.; Chaudhry, Z.T.; Al-Hendy, A. Successes and failures of uterine leiomyoma drug discovery. Expert. Opin. Drug Discov. 2018, 13, 169–177. [Google Scholar] [CrossRef]
- McLaren, J.S.; Morris, E.; Rymer, J. Gonadotrophin receptor hormone analogues in combination with add-back therapy: An update. Menopause Int. 2012, 18, 68–72. [Google Scholar] [CrossRef]
- Waibel-Treber, S.; Minne, H.W.; Scharla, S.H.; Bremen, T.H.; Ziegler, R.; Leyendecker, G. Reversible bone loss in women treated with GnRH-agonists for endometriosis and uterine leiomyoma. Hum. Reprod. 1989, 4, 384–388. [Google Scholar] [CrossRef]
- Wang, A.; Wang, S.; Owens, C.D.; Vora, J.B.; Diamond, M.P. Health Care Costs and Treatment Patterns Associated with Uterine Fibroids and Heavy Menstrual Bleeding: A Claims Analysis. J. Womens Health 2022, 31, 856–863. [Google Scholar] [CrossRef]
- Pynnä, K.; Räsänen, P.; Roine, R.P.; Vuorela, P.; Sintonen, H. Where does the money go to? Cost analysis of gynecological patients with a benign condition. PLoS ONE 2021, 16, e0254124. [Google Scholar] [CrossRef]
- Guo, X.C.; Segars, J.H. The impact and management of fibroids for fertility: An evidence-based approach. Obstet. Gynecol. Clin. N. Am. 2012, 39, 521–533. [Google Scholar] [CrossRef]
- Pongpunprut, S.; Panburana, P.; Wibulpolprasert, P.; Waiyaput, W.; Sroyraya, M.; Chansoon, T.; Sophonsritsuk, A. A Comparison of Shear Wave Elastography between Normal Myometrium, Uterine Fibroids, and Adenomyosis: A Cross-Sectional Study. Int. J. Fertil. Steril. 2022, 16, 49–54. [Google Scholar]
- Bartels, C.B.; Cayton, K.C.; Chuong, F.S.; Holthouser, K.; Mehr, S.A.; Abraham, T.; Segars, J.H. An Evidence-based Approach to the Medical Management of Fibroids: A Systematic Review. Clin. Obstet. Gynecol. 2016, 59, 30–52. [Google Scholar] [CrossRef]
- Ciebiera, M.; Ali, M.; Prince, L.; Zgliczyński, S.; Jakiel, G.; Al-Hendy, A. The Significance of Measuring Vitamin D Serum Levels in Women with Uterine Fibroids. Reprod. Sci. 2021, 28, 2098–2109. [Google Scholar] [CrossRef] [PubMed]
- Vora, Z.; Manchanda, S.; Sharma, R.; Das, C.J.; Hari, S.; Mathur, S.; Kumar, S.; Kachhawa, G.; Khan, M.A. Transvaginal Shear Wave Elastography for Assessment of Endometrial and Subendometrial Pathologies: A Prospective Pilot Study. J. Ultrasound. Med. 2022, 41, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Lin, S.; Lyu, G.R. Advances in the clinical application of ultrasound elastography in uterine imaging. Insights Imaging 2022, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Feofilova, M.A.; OPavlov, G.; Geimerling, V.E. The Effect of Life-Style and Occupational Hazards on Development of Hysteromyoma. Probl. Sotsialnoi. Gig. Zdr. Istor. Med. 2018, 26, 406–410. [Google Scholar]
- Ciebiera, M.; Esfandyari, S.; Siblini, H.; Prince, L.; Elkafas, H.; Wojtyła, C.; Al-Hendy, A.; Ali, M. Nutrition in Gynecological Diseases: Current Perspectives. Nutrients 2021, 13, 1178. [Google Scholar] [CrossRef]
- Potre, C.; Borsi, E.; Potre, O.; Ionita, I.; Samfireag, M.; Costachescu, D.; Secosan, C.; Lazar, S.; Ristescu, A.I. A Systematic Review Assessing the Impact of Vitamin D Levels on Adult Patients with Lymphoid Malignancies. Curr. Oncol. 2023, 30, 4351–4364. [Google Scholar] [CrossRef] [PubMed]
- Ostadmohammadi, V.; Milajerdi, A.; Ghayour-Mobarhan, M.; Ferns, G.; Taghizadeh, M.; Badehnoosh, B.; Mirzaei, H.; Asemi, Z. The Effects of Vitamin D Supplementation on Glycemic Control, Lipid Profiles and C-Reactive Protein Among Patients with Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr. Pharm. Des. 2019, 25, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Włodarczyk, M.; Ciebiera, M.; Zaręba, K.; Łukaszuk, K.; Jakiel, G. Vitamin D and Uterine Fibroids-Review of the Literature and Novel Concepts. Int. J. Mol. Sci. 2018, 19, 2051. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhai, Y.; Wang, C.; Liu, T.; Tian, S. Association of dietary diversity with uterine fibroids among urban premenopausal women in Shijiazhuang, China: A cross-sectional study. Asia Pac. J. Clin. Nutr. 2020, 29, 771–781. [Google Scholar]
- Guo, W.; Dai, M.; Zhong, Z.; Zhu, S.; Gong, G.; Chen, M.; Guo, J.; Zhang, Y. The association between vitamin D and uterine fibroids: A mendelian randomization study. Front. Genet. 2022, 13, 1013192. [Google Scholar] [CrossRef]
- Skowrońska, P.; Pastuszek, E.; Kuczyński, W.; Jaszczoł, M.; Kuć, P.; Jakiel, G.; Wocławek-Potocka, I.; Łukaszuk, K. The role of vitamin D in reproductive dysfunction in women—A systematic review. Ann. Agric. Environ. Med. 2016, 23, 671–676. [Google Scholar] [CrossRef]
- Xu, F.; Li, F.; Li, L.; Lin, D.; Hu, H.; Shi, Q. Vitamin D as a risk factor for the presence of asymptomatic uterine fibroids in premenopausal Han Chinese women. Fertil. Steril. 2021, 115, 1288–1293. [Google Scholar] [CrossRef]
- Tanha, F.D.; Feizabad, E.; Farahani, M.V.; Amuzegar, H.; Moradi, B.; Sadeh, S.S. The Effect of Vitamin D Deficiency on Overgrowth of Uterine Fibroids: A Blinded Randomized Clinical Trial. Int. J. Fertil. Steril. 2021, 15, 95–100. [Google Scholar]
- Al-Hendy, A.; Badr, M. Can vitamin D reduce the risk of uterine fibroids? Womens Health 2014, 10, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Rusińska, A.; Płudowski, P.; Walczak, M.; Borszewska-Kornacka, M.K.; Bossowski, A.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dobrzańska, A.; Franek, E.; Helwich, E.; et al. Vitamin D Supplementation Guidelines for General Population and Groups at Risk of Vitamin D Deficiency in Poland-Recommendations of the Polish Society of Pediatric Endocrinology and Diabetes and the Expert Panel with Participation of National Specialist Consultants and Representatives of Scientific Societies-2018 Update. Front. Endocrinol. 2018, 9, 246. [Google Scholar]
- Veldurthy, V.; Wei, R.; Oz, L.; Dhawan, P.; Jeon, Y.H.; Christakos, S. Vitamin D, calcium homeostasis and aging. Bone Res. 2016, 4, 16041. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Purohit, M.P. Which Foods Contain the Most Vitamin D? Available online: https://www.dovemed.com/healthy-living/wellness-center/which-foods-contain-most-vitamin-d/ (accessed on 22 September 2023).
- Lamberg-Allardt, C. Vitamin D in foods and as supplements. Prog. Biophys. Mol. Biol. 2006, 92, 33–38. [Google Scholar] [CrossRef]
- Orta, O.R.; Terry, K.L.; Missmer, S.A.; Harris, H.R. Dairy and related nutrient intake and risk of uterine leiomyoma: A prospective cohort study. Hum. Reprod. 2020, 35, 453–463. [Google Scholar] [CrossRef]
- Elkafas, H.; Walls, M.; Al-Hendy, A.; Ismail, N. Gut and genital tract microbiomes: Dysbiosis and link to gynecological disorders. Front. Cell Infect. Microbiol. 2022, 12, 1059825. [Google Scholar] [CrossRef]
- Judson, I.; Messiou, C. Vitamin D deficiency in the pathogenesis of leiomyoma and intravascular leiomyomatosis: A case report and review of the literature. Gynecol. Oncol. Rep. 2021, 35, 100681. [Google Scholar] [CrossRef]
- Al-Hendy, A.; Halder, S.K.; Allah, A.; Roshdy, E.; Rajaratnam, V.; Sabry, M. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: A cross-sectional observational study. Int. J. Womens Health 2013, 5, 93–100. [Google Scholar] [CrossRef]
- Baird, D.D.; Hill, M.C.; Schectman, J.M.; Hollis, B.W. Vitamin d and the risk of uterine fibroids. Epidemiology 2013, 24, 447–453. [Google Scholar] [CrossRef]
- Paffoni, A.; Somigliana, E.; Vigano’, P.; Benaglia, L.; Cardellicchio, L.; Pagliardini, L.; Papaleo, E.; Candiani, M.; Fedele, L. Vitamin D status in women with uterine leiomyomas. J. Clin. Endocrinol. Metab. 2013, 98, E1374–E1378. [Google Scholar] [CrossRef]
- Singh, V.; Barik, A.; Imam, N. Vitamin D(3) Level in Women with Uterine Fibroid: An Observational Study in Eastern Indian Population. J. Obstet. Gynaecol. India 2019, 69, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Ciavattini, A.; Carpini, G.D.; Serri, M.; Vignini, A.; Sabbatinelli, J.; Tozzi, A.; Aggiusti, A.; Clemente, N. Hypovitaminosis D and “small burden” uterine fibroids: Opportunity for a vitamin D supplementation. Medicine 2016, 95, e5698. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, R.; Tabrizi, R.; Hessami, K.; Ashari, H.; Nowrouzi-Sohrabi, P.; Hosseini-Bensenjan, M.; Asadi, N. Correlation of low serum vitamin-D with uterine leiomyoma: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2020, 18, 85. [Google Scholar] [CrossRef] [PubMed]
- Makwe, C.C.; Soibi-Harry, A.P.; Rimi, G.S.; Ugwu, O.A.; Ajayi, A.T.; Adesina, T.A.; Okunade, K.S.; Oluwole, A.A.; Anorlu, R.I. Micronutrient and Trace Element Levels in Serum of Women with Uterine Fibroids in Lagos. Cureus 2021, 13, e18638. [Google Scholar] [CrossRef]
- Nesby-O’Dell, S.; Scanlon, K.S.; Cogswell, M.E.; Gillespie, C.; Hollis, B.W.; Looker, A.C.; Allen, C.; Doughertly, C.; Gunter, E.W.; Bowman, B.A. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: Third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Clin. Nutr. 2002, 76, 187–192. [Google Scholar] [CrossRef]
- Ylikomi, T.; Laaksi, I.; Lou, Y.R.; Martikainen, P.; Miettinen, S.; Pennanen, P.; Purmonen, S.; Syvälä, H.; Vienonen, A.; Tuohimaa, P. Antiproliferative action of vitamin D. Vitam. Horm. 2002, 64, 357–406. [Google Scholar]
- Holick, M.F. Too little vitamin D in premenopausal women: Why should we care? Am. J. Clin. Nutr. 2002, 76, 3–4. [Google Scholar] [CrossRef]
- Igboeli, P.; Walker, W.; McHugh, A.; Sultan, A.; Al-Hendy, A. Burden of Uterine Fibroids: An African Perspective, A Call for Action and Opportunity for Intervention. Curr. Opin. Gynecol. Obstet. 2019, 2, 287–294. [Google Scholar]
- Kumari, R.; Nath, B.; Kashika; Gaikwad, H.S.; Sharma, M. Association between serum vitamin D level and uterine fibroid in premenopausal women in Indian population. Drug Discov. Ther. 2022, 16, 8–13. [Google Scholar] [CrossRef]
- Islam, M.S.; Akhtar, M.M.; Segars, J.H. Vitamin D deficiency and uterine fibroids: An opportunity for treatment or prevention? Fertil. Steril. 2021, 115, 1175–1176. [Google Scholar] [CrossRef]
- Halder, S.K.; Sharan, C.; Al-Hendy, O.; Al-Hendy, A. Paricalcitol, a vitamin d receptor activator, inhibits tumor formation in a murine model of uterine fibroids. Reprod. Sci. 2014, 21, 1108–1119. [Google Scholar] [CrossRef]
- Borahay, M.A.; Al-Hendy, A.; Kilic, G.S.; Boehning, D. Signaling Pathways in Leiomyoma: Understanding Pathobiology and Implications for Therapy. Mol. Med. 2015, 21, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-P.; Chen, Y.-W.; Liu, W.-H.; Chou, H.-C.; Chang, Y.-P.; Lin, S.-T.; Li, J.-M.; Jian, S.-F.; Lee, Y.-R.; Chan, H.-L. Proteomic identification of plasma biomarkers in uterine leiomyoma. Mol. Biosyst. 2012, 8, 1136–1145. [Google Scholar] [CrossRef]
- Bateman, N.W.; Tarney, C.M.; Abulez, T.S.; Hood, B.L.; Conrads, K.A.; Zhou, M.; Soltis, A.R.; Teng, P.-N.; Jackson, A.; Tian, C.; et al. Peptide ancestry informative markers in uterine neoplasms from women of European, African, and Asian ancestry. iScience 2022, 25, 103665. [Google Scholar] [CrossRef] [PubMed]
- Scharla, S.H.; Minne, H.W.; Waibel-Treber, S.; Schaible, A.; Lempert, U.G.; Wüster, C.; Leyendecker, G.; Ziegler, R. Bone mass reduction after estrogen deprivation by long-acting gonadotropin-releasing hormone agonists and its relation to pretreatment serum concentrations of 1,25-dihydroxyvitamin D3. J. Clin. Endocrinol. Metab. 1990, 70, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Combs, A.; Singh, B.; Nylander, E.; Islam, S.; Nguyen, H.V.; Parra, E.; Bello, A.; Segars, J. A Systematic Review of Vitamin D and Fibroids: Pathophysiology, Prevention, and Treatment. Reprod. Sci. 2023, 30, 1049–1064. [Google Scholar] [CrossRef] [PubMed]
- Shushan, A.; Ben-Bassat, H.; Mishani, E.; Laufer, N.; Klein, B.Y.; Rojansky, N. Inhibition of leiomyoma cell proliferation in vitro by genistein and the protein tyrosine kinase inhibitor TKS050. Fertil. Steril. 2007, 87, 127–135. [Google Scholar] [CrossRef]
- ElHusseini, H.; Elkafas, H.; Abdelaziz, M.; Halder, S.; Atabiekov, I.; Eziba, N.; Ismail, N.; El Andaloussi, A.; Al-Hendy, A. Diet-induced vitamin D deficiency triggers inflammation and DNA damage profile in murine myometrium. Int. J. Womens Health 2018, 10, 503–514. [Google Scholar] [CrossRef]
- AlAshqar, A.; Reschke, L.; Kirschen, G.W.; Borahay, M.A. Role of inflammation in benign gynecologic disorders: From pathogenesis to novel therapies. Biol. Reprod. 2021, 105, 7–31. [Google Scholar] [CrossRef]
- Jukic, A.M.; Steiner, A.Z.; Baird, D.D. Lower plasma 25-hydroxyvitamin D is associated with irregular menstrual cycles in a cross-sectional study. Reprod. Biol. Endocrinol. 2015, 13, 20. [Google Scholar] [CrossRef]
- Brakta, S.; Diamond, J.S.; Al-Hendy, A.; Diamond, M.P.; Halder, S.K. Role of vitamin D in uterine fibroid biology. Fertil. Steril. 2015, 104, 698–706. [Google Scholar] [CrossRef]
- Halder, S.K.; Osteen, K.G.; Al-Hendy, A. Vitamin D3 inhibits expression and activities of matrix metalloproteinase-2 and -9 in human uterine fibroid cells. Hum. Reprod. 2013, 28, 2407–2416. [Google Scholar] [CrossRef]
- Bläuer, M.; Rovio, P.H.; Ylikomi, T.; Heinonen, P.K. Vitamin D inhibits myometrial and leiomyoma cell proliferation in vitro. Fertil. Steril. 2009, 91, 1919–1925. [Google Scholar] [CrossRef]
- Corachán, A.; Ferrero, H.; Escrig, J.; Monleon, J.; Faus, A.; Cervelló, I.; Pellicer, A. Long-term vitamin D treatment decreases human uterine leiomyoma size in a xenograft animal model. Fertil. Steril. 2020, 113, 205–216.e4. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, E.; Piltan, S.; Gholami, M.; Akbari, M.; Falahati, Z.; Yassaee, F.; Sadeghi, H.; Mirfakhraie, R. CYP24A1 expression analysis in uterine leiomyoma regarding MED12 mutation profile. Arch. Gynecol. Obstet. 2021, 303, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Wrzosek, M.; Wojtyła, C.; Zaręba, K.; Nowicka, G.; Jakiel, G.; Włodarczyk, M. Vitamin D receptor gene polymorphisms and uterine fibroid incidence in Caucasian women. Arch. Med. Sci. 2021, 17, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Mantell, D.J.; Owens, P.E.; Bundred, N.J.; Mawer, E.B.; Canfield, A.E. 1 alpha,25-dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circ. Res. 2000, 87, 214–220. [Google Scholar] [CrossRef]
- Stewart, E.A.; Nowak, R.A. Uterine Fibroids: Hiding in Plain Sight. Physiology 2022, 37, 16–27. [Google Scholar] [CrossRef]
- Jain, V.; Chodankar, R.R.; Maybin, J.A.; Critchley, H.O.D. Uterine bleeding: How understanding endometrial physiology underpins menstrual health. Nat. Rev. Endocrinol. 2022, 18, 290–308. [Google Scholar] [CrossRef]
- Szydłowska, I.; Nawrocka-Rutkowska, J.; Brodowska, A.; Marciniak, A.; Starczewski, A.; Szczuko, M. Dietary Natural Compounds and Vitamins as Potential Cofactors in Uterine Fibroids Growth and Development. Nutrients 2022, 14, 734. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Shahin, S.M.; Sabri, N.A.; Al-Hendy, A.; Yang, Q. Hypovitaminosis D exacerbates the DNA damage load in human uterine fibroids, which is ameliorated by vitamin D3 treatment. Acta Pharmacol. Sin. 2019, 40, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Elkafas, H.; Ali, M.; Elmorsy, E.; Kamel, R.; Thompson, W.E.; Badary, O.; Al-Hendy, A.; Yang, Q. Vitamin D3 Ameliorates DNA Damage Caused by Developmental Exposure to Endocrine Disruptors in the Uterine Myometrial Stem Cells of Eker Rats. Cells 2020, 9, 1459. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Baek, M.S.; Yoon, D.S.; Park, J.S.; Yoon, B.W.; Oh, B.S.; Park, J.; Kim, H.J. Vitamin D Inhibits Expression and Activity of Matrix Metalloproteinase in Human Lung Fibroblasts (HFL-1) Cells. Tuberc. Respir. Dis. 2014, 77, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; KOsteen, G.; Al-Hendy, A. 1,25-dihydroxyvitamin d3 reduces extracellular matrix-associated protein expression in human uterine fibroid cells. Biol. Reprod. 2013, 89, 150. [Google Scholar] [CrossRef]
- Vergara, D.; Catherino, W.H.; Trojano, G.; Tinelli, A. Vitamin D: Mechanism of Action and Biological Effects in Uterine Fibroids. Nutrients 2021, 13, 597. [Google Scholar] [CrossRef]
- Ali, M.; Shahin, S.M.; Sabri, N.A.; Al-Hendy, A.; Yang, Q. 1,25 Dihydroxyvitamin D3 Enhances the Antifibroid Effects of Ulipristal Acetate in Human Uterine Fibroids. Reprod. Sci. 2019, 26, 812–828. [Google Scholar] [CrossRef]
- Ciebiera, M.; Włodarczyk, M.; Słabuszewska-Jóźwiak, A.; Nowicka, G.; Jakiel, G. Influence of vitamin D and transforming growth factor β3 serum concentrations, obesity, and family history on the risk for uterine fibroids. Fertil. Steril. 2016, 106, 1787–1792. [Google Scholar] [CrossRef]
- Halder, S.; Al-Hendy, A. Hypovitaminosis D and high serum transforming growth factor beta-3: Important biomarkers for uterine fibroids risk. Fertil. Steril. 2016, 106, 1648–1649. [Google Scholar] [CrossRef]
- Halder, S.K.; JGoodwin, S.; Al-Hendy, A. 1,25-Dihydroxyvitamin D3 reduces TGF-beta3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J. Clin. Endocrinol. Metab. 2011, 96, E754–E762. [Google Scholar] [CrossRef] [PubMed]
- Corachán, A.; Trejo, M.G.; Carbajo-García, M.C.; Monleón, J.; Escrig, J.; Faus, A.; Pellicer, A.; Cervelló, I.; Ferrero, H. Vitamin D as an effective treatment in human uterine leiomyomas independent of mediator complex subunit 12 mutation. Fertil. Steril. 2021, 115, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Corachán, A.; Ferrero, H.; Aguilar, A.; Garcia, N.; Monleon, J.; Faus, A.; Cervelló, I.; Pellicer, A. Inhibition of tumor cell proliferation in human uterine leiomyomas by vitamin D via Wnt/β-catenin pathway. Fertil. Steril. 2019, 111, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Al-Hendy, A.; Diamond, M.P.; Boyer, T.G.; Halder, S.K. Vitamin D3 Inhibits Wnt/β-Catenin and mTOR Signaling Pathways in Human Uterine Fibroid Cells. J. Clin. Endocrinol. Metab. 2016, 101, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Arjeh, S.; Darsareh, F.; Asl, Z.A.; Kutenaei, M.A. Effect of oral consumption of vitamin D on uterine fibroids: A randomized clinical trial. Complement Ther. Clin. Pract. 2020, 39, 101159. [Google Scholar] [CrossRef]
- Hajhashemi, M.; Ansari, M.; Haghollahi, F.; Eslami, B. The effect of vitamin D supplementation on the size of uterine leiomyoma in women with vitamin D deficiency. Casp. J. Intern. Med. 2019, 10, 125–131. [Google Scholar]
- Halder, S.K.; Sharan, C.; Al-Hendy, A. 1,25-dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the Eker rat model. Biol. Reprod. 2012, 86, 116. [Google Scholar] [CrossRef] [PubMed]
- Harmon, Q.E.; Patchel, S.A.; Denslow, S.; LaPorte, F.; Cooper, T.; Wise, L.A.; Wegienka, G.; Baird, D.D. Vitamin D and uterine fibroid growth, incidence, and loss: A prospective ultrasound study. Fertil. Steril. 2022, 118, 1127–1136. [Google Scholar] [CrossRef]
- Vahdat, M.; Allahqoli, L.; Mirzaei, H.; Giovannucci, E.; Salehiniya, H.; Mansouri, G.; Alkatout, I. The effect of vitamin D on recurrence of uterine fibroids: A randomized, double-blind, placebo-controlled pilot study. Complement Ther. Clin. Pract. 2022, 46, 101536. [Google Scholar] [CrossRef]
- Wong, J.Y.; Gold, E.B.; Johnson, W.O.; Lee, J.S. Circulating Sex Hormones and Risk of Uterine Fibroids: Study of Women’s Health Across the Nation (SWAN). J. Clin. Endocrinol. Metab. 2016, 101, 123–130. [Google Scholar] [CrossRef]
- Harmon, Q.E.; Umbach, D.M.; Baird, D.D. Use of Estrogen-Containing Contraception Is Associated with Increased Concentrations of 25-Hydroxy Vitamin D. J. Clin. Endocrinol. Metab. 2016, 101, 3370–3377. [Google Scholar] [CrossRef]
- Borahay, M.A.; Asoglu, M.R.; Mas, A.; Adam, S.; Kilic, G.S.; Al-Hendy, A. Estrogen Receptors and Signaling in Fibroids: Role in Pathobiology and Therapeutic Implications. Reprod. Sci. 2017, 24, 1235–1244. [Google Scholar] [CrossRef]
- Al-Hendy, A.; Diamond, M.P.; El-Sohemy, A.; Halder, S.K. 1,25-dihydroxyvitamin D3 regulates expression of sex steroid receptors in human uterine fibroid cells. J. Clin. Endocrinol. Metab. 2015, 100, E572–E582. [Google Scholar] [CrossRef] [PubMed]
- Purusothaman, V.; Young, S.L. Vitamin D and uterine leiomyomata: Is it time to let the sunshine in? Fertil. Steril. 2021, 115, 340–341. [Google Scholar] [CrossRef]
- Budani, M.C.; Fensore, S.; DI Marzio, M.; Tiboni, G.M. Effect of vitamin D supplementation on uterine fibroids: A meta-analysis of the literature. Minerva Obstet. Gynecol. 2022, 74, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Cross, C.I.; Driggers, P.H.; McCarthy, B.E.; Diab, M.; Brennan, J.; Segars, J.H. A-kinase anchoring protein 13 interacts with the vitamin D receptor to alter vitamin D-dependent gene activation in uterine leiomyoma cells. F&S Sci. 2021, 2, 303–314. [Google Scholar]
- Güleç Yılmaz, S.; Gül, T.; Attar, R.; Yıldırım, G.; Işbir, T. Association between fok1 polymorphism of vitamin D receptor gene with uterine leiomyoma in Turkish populations. J. Turk. Ger. Gynecol. Assoc. 2018, 19, 128–131. [Google Scholar] [CrossRef]
- Wise, L.A.; Ruiz-Narváez, E.A.; Haddad, S.A.; Rosenberg, L.; Palmer, J.R. Polymorphisms in vitamin D-related genes and risk of uterine leiomyomata. Fertil. Steril. 2014, 102, 503–510.e1. [Google Scholar] [CrossRef]
- Li, S.; Chen, B.; Sheng, B.; Wang, J.; Zhu, X. The associations between serum vitamin D, calcium and uterine fibroids in Chinese women: A case-controlled study. J. Int. Med. Res. 2020, 48, 0300060520923492. [Google Scholar] [CrossRef]
- Miriello, D.; Galanti, F.; Cignini, P.; Antonaci, D.; Schiavi, M.C.; Rago, R. Uterine fibroids treatment: Do we have new valid alternative? Experiencing the combination of vitamin D plus epigallocatechin gallate in childbearing age affected women. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2843–2851. [Google Scholar]
- Aninye, I.O.; Laitner, M.H. Uterine Fibroids: Assessing Unmet Needs from Bench to Bedside. J. Womens Health 2021, 30, 1060–1067. [Google Scholar] [CrossRef]
- Suneja, A.; Faridi, F.; Bhatt, S.; Guleria, K.; Mehndiratta, M.; Sharma, R. Effect of Vitamin D3 Supplementation on Symptomatic Uterine Leiomyoma in Women with Hypovitaminosis D. J. Midlife Health 2021, 12, 53–60. [Google Scholar] [CrossRef]
- Kaplan, Z.A.O.; Taşçi, Y.; Topçu, H.O.; Erkaya, S. 25-Hydroxy vitamin D levels in premenopausal Turkish women with uterine leiomyoma. Gynecol. Endocrinol. 2018, 34, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.S.O.; da Silva, B.B.; de Medeiros, M.L.; dos Santos, A.R.; Brazil, E.D.D.N.; Filho, W.M.N.E.; Ibiapina, J.O.; Brito, A.G.A.; Costa, P.V.L. Evaluation of vitamin D receptor expression in uterine leiomyoma and nonneoplastic myometrial tissue: A cross-sectional controlled study. Reprod. Biol. Endocrinol. 2021, 19, 67. [Google Scholar] [CrossRef] [PubMed]
- Płudowski, P.; Kos-Kudła, B.; Walczak, M.; Fal, A.; Zozulińska-Ziółkiewicz, D.; Sieroszewski, P.; Peregud-Pogorzelski, J.; Lauterbach, R.; Targowski, T.; Lewiński, A.; et al. Guidelines for Preventing and Treating Vitamin D Deficiency: A 2023 Update in Poland. Nutrients 2023, 15, 695. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef]
- Porcaro, G.; Santamaria, A.; Giordano, D.; Angelozzi, P. Vitamin D plus epigallocatechin gallate: A novel promising approach for uterine myomas. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3344–3351. [Google Scholar]
- Sheng, B.; Song, Y.; Liu, Y.; Jiang, C.; Zhu, X. Association between vitamin D and uterine fibroids: A study protocol of an open-label, randomised controlled trial. BMJ Open 2020, 10, e038709. [Google Scholar] [CrossRef]
- Grundmann, M.; von Versen-Höynck, F. Vitamin D—Roles in women’s reproductive health? Reprod. Biol. Endocrinol. 2011, 9, 146. [Google Scholar] [CrossRef]
- Várbíró, S.; Takács, I.; Tűű, L.; Nas, K.; Sziva, R.E.; Hetthéssy, J.R.; Török, M. Effects of Vitamin D on Fertility, Pregnancy and Polycystic Ovary Syndrome-A Review. Nutrients 2022, 14, 1649. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Y.; Xu, Y. Vitamin D deficiency in pregnant women: Influenced by multiple risk factors and increase the risks of spontaneous abortion and small-for-gestational age. Medicine 2021, 100, e27505. [Google Scholar] [CrossRef]
- Tunau, K.A.; Garba, J.A.; Panti, A.A.; Shehu, C.E.; Adamu, A.N.; AbdulRahman, M.B.; Ahmad, M.K. Low plasma vitamin D as a predictor of uterine fibroids in a nigerian population. Niger. Postgrad Med. J. 2021, 28, 181–186. [Google Scholar] [CrossRef]
- Srivastava, P.; Gupta, H.P.; Singhi, S.; Khanduri, S.; Rathore, B. Evaluation of 25-hydroxy vitamin D3 levels in patients with a fibroid uterus. J. Obstet. Gynaecol. 2020, 40, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; AlAshqar, A.; El Sabeh, M.; Miyashita-Ishiwata, M.; Reschke, L.; Brennan, J.T.; Fader, A.; Borahay, M.A. Diet and Nutrition in Gynecological Disorders: A Focus on Clinical Studies. Nutrients 2021, 13, 1747. [Google Scholar] [CrossRef] [PubMed]
- Kamal, D.A.M.; Salamt, N.; Zaid, S.S.M.; Mokhtar, M.H. Beneficial Effects of Green Tea Catechins on Female Reproductive Disorders: A Review. Molecules 2021, 26, 2675. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Lu, J.-L.; Liang, Y.-R.; Li, Q.-S. Suppressive Effects of EGCG on Cervical Cancer. Molecules 2018, 23, 2334. [Google Scholar] [CrossRef]
- Chen, X.; Man, G.C.W.; Hung, S.W.; Zhang, T.; Fung, L.W.Y.; Cheung, C.W.; Chung, J.P.W.; Li, T.C.; Wang, C.C. Therapeutic effects of green tea on endometriosis. Crit. Rev. Food Sci. Nutr. 2023, 63, 3222–3235. [Google Scholar] [CrossRef]
- Kao, Y.H.; Hiipakka, R.A.; Liao, S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology 2000, 141, 980–987. [Google Scholar] [CrossRef]
- Zhang, D.; Al-Hendy, M.; Richard-Davis, G.; Montgomery-Rice, V.; Sharan, C.; Rajaratnam, V.; Khurana, A.; Al-Hendy, A. Green tea extract inhibits proliferation of uterine leiomyoma cells in vitro and in nude mice. Am. J. Obstet. Gynecol. 2010, 202, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults—Results of a systematic review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef] [PubMed]
- Arip, M.; Yap, V.L.; Rajagopal, M.; Selvaraja, M.; Dharmendra, K.; Chinnapan, S. Evidence-Based Management of Uterine Fibroids with Botanical Drugs-A Review. Front. Pharmacol. 2022, 13, 878407. [Google Scholar] [CrossRef]
- Zhang, D.; Al-Hendy, M.; Richard-Davis, G.; Montgomery-Rice, V.; Rajaratnam, V.; Al-Hendy, A. Antiproliferative and proapoptotic effects of epigallocatechin gallate on human leiomyoma cells. Fertil. Steril. 2010, 94, 1887–1893. [Google Scholar] [CrossRef]
- Zhang, D.; Rajaratnam, V.; Al-Hendy, O.; Halder, S.; Al-Hendy, A. Green tea extract inhibition of human leiomyoma cell proliferation is mediated via catechol-O-methyltransferase. Gynecol. Obstet. Investig. 2014, 78, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.S.; Soave, C.; Edbauer, T.G.; Patel, K.R.; Elghoul, Y.; de Oliveira, A.V.; Renzetti, A.; Foldes, R.; Chan, T.H.; Dou, Q.P. Discovery of Green Tea Polyphenol-Based Antitumor Drugs: Mechanisms of Action and Clinical Implications. In Medicinal Plants: From Farm to Pharmacy; Joshee, N., Dhekney, S.A., Parajuli, P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 313–332. [Google Scholar]
- Kondo, A.; Takeda, T.; Li, B.; Tsuiji, K.; Kitamura, M.; Wong, T.F.; Yaegashi, N. Epigallocatechin-3-gallate potentiates curcumin’s ability to suppress uterine leiomyosarcoma cell growth and induce apoptosis. Int. J. Clin. Oncol. 2013, 18, 380–388. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef]
- Sabanayagam, R.; Krishnamoorthy, S.; Gnanagurusamy, J.; Muruganatham, B.; Muthusami, S. EGCG attenuate EGF triggered matrix abundance and migration in HPV positive and HPV negative cervical cancer cells. Med. Oncol. 2023, 40, 261. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xie, Y.; Feng, Y.; Zhang, L.; Huang, X.; Shen, X.; Luo, X. (-)-Epigallocatechingallate induces apoptosis in B lymphoma cells via caspase-dependent pathway and Bcl-2 family protein modulation. Int. J. Oncol. 2015, 46, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.E.; Jung, B.H.; Fiorino, A.; Gomez, J.; Del Rosario, E.; Cabrera, B.L.; Huang, S.C.; Chow, J.Y.C.; Carethers, J.M.; Ji, T.; et al. Bone morphogenetic protein signaling and growth suppression in colon cancer. Am. J. Physiol. Gastrointest Liver Physiol. 2006, 291, G135–G145. [Google Scholar] [CrossRef]
- Horvath, L.G.; Henshall, S.M.; Kench, J.G.; Turner, J.J.; Golovsky, D.; Brenner, P.C.; O’Neill, G.F.; Kooner, R.; Stricker, P.D.; Grygiel, J.J.; et al. Loss of BMP2, Smad8, and Smad4 expression in prostate cancer progression. Prostate 2004, 59, 234–242. [Google Scholar] [CrossRef]
- Leong, H.; Mathur, P.S.; Greene, G.L. Green tea catechins inhibit angiogenesis through suppression of STAT3 activation. Breast Cancer Res. Treat. 2009, 117, 505–515. [Google Scholar] [CrossRef]
- Jung, Y.D.; Ellis, L.M. Inhibition of tumour invasion and angiogenesis by epigallocatechin gallate (EGCG), a major component of green tea. Int. J. Exp. Pathol. 2001, 82, 309–316. [Google Scholar] [CrossRef]
- Islam, M.S.; Akhtar, M.M.; Ciavattini, A.; Giannubilo, S.R.; Protic, O.; Janjusevic, M.; Procopio, A.D.; Segars, J.H.; Castellucci, M.; Ciarmela, P. Use of dietary phytochemicals to target inflammation, fibrosis, proliferation, and angiogenesis in uterine tissues: Promising options for prevention and treatment of uterine fibroids? Mol. Nutr. Food Res. 2014, 58, 1667–1684. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Ciebiera, M.; Vafaei, S.; Alkhrait, S.; Chen, H.-Y.; Chiang, Y.-F.; Huang, K.-C.; Feduniw, S.; Hsia, S.-M.; Al-Hendy, A. Progesterone Signaling and Uterine Fibroid Pathogenesis; Molecular Mechanisms and Potential Therapeutics. Cells 2023, 12, 1117. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Zhu, K.; Liu, Z.; Huang, J. Prooxidant Effects of Epigallocatechin-3-Gallate in Health Benefits and Potential Adverse Effect. Oxid. Med. Cell Longev. 2020, 2020, 9723686. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect Biol. 2011, 3, a005058. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Parish, M.; Brennan, J.T.; Winer, B.L.; Segars, J.H. Targeting fibrotic signaling pathways by EGCG as a therapeutic strategy for uterine fibroids. Sci. Rep. 2023, 13, 8492. [Google Scholar] [CrossRef] [PubMed]
- Goodin, M.G.; Fertuck, K.C.; Zacharewski, T.R.; Rosengren, R.J. Estrogen receptor-mediated actions of polyphenolic catechins in vivo and in vitro. Toxicol. Sci. 2002, 69, 354–361. [Google Scholar] [CrossRef]
- Payne, A.; Taka, E.; Adinew, G.M.; Soliman, K.F.A. Molecular Mechanisms of the Anti-Inflammatory Effects of Epigallocatechin 3-Gallate (EGCG) in LPS-Activated BV-2 Microglia Cells. Brain Sci. 2023, 13, 632. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Ma, Y.; Liu, Y.; Lin, C.-C.; Li, S.; Zhan, J.; Ho, C.-T. Immunomodulatory Effects of Green Tea Polyphenols. Molecules 2021, 26, 3755. [Google Scholar] [CrossRef]
- Xia, S.; Ou, K.; Zhang, S.; Huang, J.; Fang, L.; Wang, C.; Wang, Q. EGCG exposure during pregnancy affects uterine histomorphology in F1 female mice and the underlying mechanisms. Food Chem. Toxicol. 2022, 167, 113306. [Google Scholar] [CrossRef]
- Kramer, K.J.; Ottum, S.; Gonullu, D.; Bell, C.; Ozbeki, H.; Berman, J.M.; Recanati, M.-A. Reoperation rates for recurrence of fibroids after abdominal myomectomy in women with large uterus. PLoS ONE 2021, 16, e0261085. [Google Scholar] [CrossRef]
- Li, Z.-L.; Huang, T.-Y.; Ho, Y.; Shih, Y.-J.; Chen, Y.-R.; Tang, H.-Y.; Lin, H.-Y.; Whang-Peng, J.; Wang, K. Herbal Medicine in Uterine Fibroid; Intechopen: London, UK, 2020. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Tipoe, G.L.; Leung, T.-M.; Hung, M.-W.; Fung, M.-L. Green Tea Polyphenols as an Anti-Oxidant and Anti-Inflammatory Agent for Cardiovascular Protection. Cardiovasc. Hematol. Disord. Targets 2007, 7, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic Effects of Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. Int. J. Mol. Sci. 2022, 24, 340. [Google Scholar] [CrossRef] [PubMed]
- Hazimeh, D.; Massoud, G.; Parish, M.; Singh, B.; Segars, J.; Islam, M.S. Green Tea and Benign Gynecologic Disorders: A New Trick for An Old Beverage? Nutrients 2023, 15, 1439. [Google Scholar] [CrossRef] [PubMed]
- Ozercan, I.H.; Sahin, N.; Akdemir, F.; Onderci, M.; Seren, S.; Sahin, K.; Kucuk, O. Chemoprevention of fibroid tumors by [-]-epigallocatechin-3-gallate in quail. Nutr. Res. 2008, 28, 92–97. [Google Scholar] [CrossRef]
- Ferrari, E.; Bettuzzi, S.; Naponelli, V. The Potential of Epigallocatechin Gallate (EGCG) in Targeting Autophagy for Cancer Treatment: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 6075. [Google Scholar] [CrossRef]
- Hill, A. EGCG (Epigallocatechin Gallate): Benefits, Dosage, and Safety. 2019. Available online: https://www.healthline.com/nutrition/egcg-epigallocatechin-gallate (accessed on 22 September 2023).
- Biro, R.; Richter, R.; Ortiz, M.; Sehouli, J.; David, M. Effects of epigallocatechin gallate-enriched green tea extract capsules in uterine myomas: Results of an observational study. Arch. Gynecol. Obstet. 2021, 303, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, Y.; Ling, F.; Guan, Y.; Zhang, D.; Zhu, Q.; Liu, J.; Wu, Y.; Niu, Y. The phytochemical epigallocatechin gallate prolongs the lifespan by improving lipid metabolism, reducing inflammation and oxidative stress in high-fat diet-fed obese rats. Aging Cell 2020, 19, e13199. [Google Scholar] [CrossRef]
- Chhacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef]
- Siblini, H.; Al-Hendy, A.; Segars, J.; González, F.; Taylor, H.S.; Singh, B.; Flaminia, A.; Flores, V.A.; Christman, G.M.; Huang, H.; et al. Assessing the Hepatic Safety of Epigallocatechin Gallate (EGCG) in Reproductive-Aged Women. Nutrients 2023, 15, 320. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Męczekalski, B.; Łukaszuk, K.; Jakiel, G. Potential synergism between ulipristal acetate and vitamin D3 in uterine fibroid pharmacotherapy—2 case studies. Gynecol. Endocrinol. 2019, 35, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Łukaszuk, K.; Męczekalski, B.; Ciebiera, M.; Wojtyła, C.; Słabuszewska-Jóźwiak, A.; Jakiel, G. Alternative Oral Agents in Prophylaxis and Therapy of Uterine Fibroids-An Up-to-Date Review. Int. J. Mol. Sci. 2017, 18, 2586. [Google Scholar] [CrossRef]
- Tinelli, A.; Gustapane, S.; D’oria, O.; Licchelli, M.; Panese, G. Nutraceuticals in fibroid management after ulipristal acetate administration: An observational study on patients’ compliance. Int. J. Gynaecol. Obstet. 2022, 156, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Grandi, G.; Del Savio, M.C.; Melotti, C.; Feliciello, L.; Facchinetti, F. Vitamin D and green tea extracts for the treatment of uterine fibroids in late reproductive life: A pilot, prospective, daily-diary based study. Gynecol. Endocrinol. 2022, 38, 63–67. [Google Scholar] [CrossRef]
- Mitro, S.D.; Zota, A.R. Vitamin D and uterine leiomyoma among a sample of US women: Findings from NHANES, 2001–2006. Reprod. Toxicol. 2015, 57, 81–86. [Google Scholar] [CrossRef]
- Mohamad Zaid, S.S.; Kassim, N.M.; Othman, S. Tualang Honey Protects against BPA-Induced Morphological Abnormalities and Disruption of ERα, ERβ, and C3 mRNA and Protein Expressions in the Uterus of Rats. Evid. Based Complement Alternat. Med. 2015, 2015, 202874. [Google Scholar] [CrossRef]
- Tabrizian, K.; Shokouhinia, R.; Tanha, F.D.; Ghaemi, M.; Ghajarzadeh, M.; Shahraki, Z. Effect of Two Different Doses of Vitamin D Supplementation on Uterine Myoma on South East Iranian Population: A Clinical Trial. J. Family Reprod. Health 2021, 15, 248–251. [Google Scholar] [CrossRef]
- Bariani, M.V.; Rangaswamy, R.; Siblini, H.; Yang, Q.; Al-Hendy, A.; Zota, A.R. The role of endocrine-disrupting chemicals in uterine fibroid pathogenesis. Curr. Opin. Endocrinol. Diabetes 2020, 27, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yu, L.; Castro, L.; Moore, A.B.; Hermon, T.; Bortner, C.; Sifre, M.; Dixon, D. An endocrine-disrupting chemical, fenvalerate, induces cell cycle progression and collagen type I expression in human uterine leiomyoma and myometrial cells. Toxicol. Lett. 2010, 196, 133–141. [Google Scholar] [CrossRef]
- Lee, G.; Kim, S.; Bastiaensen, M.; Malarvannan, G.; Poma, G.; Casero, N.C.; Gys, C.; Covaci, A.; Lee, S.; Lim, J.-E.; et al. Exposure to organophosphate esters, phthalates, and alternative plasticizers in association with uterine fibroids. Environ. Res. 2020, 189, 109874. [Google Scholar] [CrossRef]
- Jeong, E.H.; Hong, G.Y.; Kim, B.R.; Park, S.N.; Lee, H.-H.; Na, Y.-J.; Namkung, J. The Relationship between Uterine Myoma Growth and the Endocrine Disruptor in Postmenopausal Women. J. Menopausal Med. 2013, 19, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Prusinski, L.; Al-Hendy, A.; Yang, Q. Developmental exposure to endocrine disrupting chemicals alters the epigenome: Identification of reprogrammed targets. Gynecol. Obstet. Res. 2016, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bariani, M.V.; Cui, Y.H.; Ali, M.; Bai, T.; Grimm, S.L.; Coarfa, C.; Walker, C.L.; He, Y.Y.; Yang, Q.; Al-Hendy, A. TGFbeta signaling links early-life endocrine-disrupting chemicals exposure to suppression of nucleotide excision repair in rat myometrial stem cells. Res. Sq. 2023; in press. [Google Scholar]
- Prusinski Fernung, L.E.; Yang, Q.; Sakamuro, D.; Kumari, A.; Mas, A.; Al-Hendy, A. Endocrine disruptor exposure during development increases incidence of uterine fibroids by altering DNA repair in myometrial stem cells. Biol. Reprod. 2018, 99, 735–748. [Google Scholar] [CrossRef]
- Li, Z.; Yin, H.; Chen, K.; Ding, B.; Xu, J.; Ren, M.; Zhang, C.; Shen, Y. Effects of bisphenol A on uterine leiomyoma: In vitro and in vivo evaluation with mechanistic insights related to XBP1. Ecotoxicol. Environ. Saf. 2022, 247, 114201. [Google Scholar] [CrossRef] [PubMed]
- Katz, T.A.; Yang, Q.; Treviño, L.S.; Walker, C.L.; Al-Hendy, A. Endocrine-disrupting chemicals and uterine fibroids. Fertil. Steril. 2016, 106, 967–977. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Y.; Ma, H.; Xu, Q.; Wu, X. Combined Exposure to Multiple Endocrine Disruptors and Uterine Leiomyomata and Endometriosis in US Women. Front. Endocrinol. 2021, 12, 726876. [Google Scholar] [CrossRef]
- Laws, M.J.; Neff, A.M.; Brehm, E.; Warner, G.R.; Flaws, J.A. Endocrine disrupting chemicals and reproductive disorders in women, men, and animal models. Adv. Pharmacol. 2021, 92, 151–190. [Google Scholar]
- Pacyga, D.C.; Ryva, B.A.; Nowak, R.A.; Bulun, S.E.; Yin, P.; Li, Z.; Flaws, J.A.; Strakovsky, R.S. Midlife Urinary Phthalate Metabolite Concentrations and Prior Uterine Fibroid Diagnosis. Int. J. Environ. Res. Public Health 2022, 19, 2741. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, Y.; Mok, S.; Choi, K.; Park, J.; Moon, H.-B.; Choi, G.; Kim, H.-J.; Kim, S.Y.; Choi, S.R.; et al. Associations of exposure to phthalates and environmental phenols with gynecological disorders. Reprod. Toxicol. 2020, 95, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Chang, C.H.; Lee, J.S.; Kim, Y.J.; Kim, M.D.; Han, D.W. Comparison of the efficacy of dexmedetomidine plus fentanyl patient-controlled analgesia with fentanyl patient-controlled analgesia for pain control in uterine artery embolization for symptomatic fibroid tumors or adenomyosis: A prospective, randomized study. J. Vasc. Interv. Radiol. 2013, 24, 779–786. [Google Scholar]
- Hodges, L.C.; Hunter, D.S.; Bergerson, J.S.; Fuchs-Young, R.; Walker, C.L. An in vivo/in vitro model to assess endocrine disrupting activity of xenoestrogens in uterine leiomyoma. Ann. N. Y. Acad. Sci. 2001, 948, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Ali, M.; Treviño, L.S.; Mas, A.; Ismail, N.; Al-Hendy, A. Epigenetic Modulation of Inflammatory Pathways in Myometrial Stem Cells and Risk of Uterine Fibroids. Int. J. Mol. Sci. 2023, 24, 11641. [Google Scholar] [CrossRef] [PubMed]
- Rumph, J.T.; Stephens, V.R.; Martin, J.L.; Brown, L.K.; Thomas, P.L.; Cooley, A.; Osteen, K.G.; Bruner-Tran, K.L. Uncovering Evidence: Associations between Environmental Contaminants and Disparities in Women’s Health. Int. J. Environ. Res. Public Health 2022, 19, 1257. [Google Scholar] [CrossRef]
- Mas, A.; Stone, L.; O’Connor, P.M.; Yang, Q.; Kleven, D.; Simon, C.; Walker, C.L.; Al-Hendy, A. Developmental Exposure to Endocrine Disruptors Expands Murine Myometrial Stem Cell Compartment as a Prerequisite to Leiomyoma Tumorigenesis. Stem Cells 2017, 35, 666–678. [Google Scholar] [CrossRef]
- Bethea, T.N.; Wesselink, A.K.; Weuve, J.; McClean, M.D.; Hauser, R.; Williams, P.L.; Ye, X.; Calafat, A.M.; Baird, D.D.; Wise, L.A. Correlates of exposure to phenols, parabens, and triclocarban in the Study of Environment, Lifestyle and Fibroids. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 117–136. [Google Scholar] [CrossRef]
- Cho, Y.J.; Yun, J.H.; Kim, S.J.; Kwon, H.Y. Nonpersistent endocrine disrupting chemicals and reproductive health of women. Obstet. Gynecol. Sci. 2020, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Crain, D.A.; Janssen, S.J.; Edwards, T.M.; Heindel, J.; Ho, S.-M.; Hunt, P.; Iguchi, T.; Juul, A.; McLachlan, J.A.; Schwartz, J.; et al. Female reproductive disorders: The roles of endocrine-disrupting compounds and developmental timing. Fertil. Steril. 2008, 90, 911–940. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Ali, M.; Zgliczyńska, M.; Skrzypczak, M.; Al-Hendy, A. Vitamins and Uterine Fibroids: Current Data on Pathophysiology and Possible Clinical Relevance. Int. J. Mol. Sci. 2020, 21, 5528. [Google Scholar] [CrossRef] [PubMed]
- Seli, D.A.; Taylor, H.S. The impact of air pollution and endocrine disruptors on reproduction and assisted reproduction. Curr Opin Obstet. Gynecol. 2023, 35, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Hodges, R.E.; Minich, D.M. Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J. Nutr. Metab. 2015, 2015, 760689. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.P.; Kim, H.; Wong, E.; Rebholz, C.M. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013–2014. Environ. Int. 2019, 131, 105057. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.E.; Braun, J.; Trasande, L.; Dills, R.; Sathyanarayana, S. Phthalates and diet: A review of the food monitoring and epidemiology data. Environ. Health 2014, 13, 43. [Google Scholar] [CrossRef]
- Russ, K. A Review of the Evidence: Health Effects of Personal Care Products. Nurs. Women’s Health 2009, 13, 392–401. [Google Scholar] [CrossRef]
- Martin, L.; Zhang, Y.; First, O.; Mustieles, V.; Dodson, R.; Rosa, G.; Coburn-Sanderson, A.; Adams, C.D.; Messerlian, C. Lifestyle interventions to reduce endocrine-disrupting phthalate and phenol exposures among reproductive age men and women: A review and future steps. Environ. Int. 2022, 170, 107576. [Google Scholar] [CrossRef]
- Rouillon, S.; El Ouazzani, H.; Hardouin, J.B.; Enjalbert, L.; Rabouan, S.; Migeot, V.; Albouy-Llaty, M. How to Educate Pregnant Women about Endocrine Disruptors? Int. J. Environ. Res. Public Health 2020, 17, 2156. [Google Scholar] [CrossRef]
- Haruty, B.; Friedman, J.; Hopp, S.; Daniels, R.; Pregler, J. Reproductive health and the environment: Counseling patients about risks. Cleveland Clin. J. Med. 2016, 83, 367. [Google Scholar] [CrossRef]
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, J. Phthalates: Toxicology and exposure. Int. J. Hyg. Environ. Health 2007, 210, 623–634. [Google Scholar] [CrossRef]
- Xie, H.; Han, W.; Xie, Q.; Xu, T.; Zhu, M.; Chen, J. Face mask-A potential source of phthalate exposure for human. J. Hazard Mater. 2022, 422, 126848. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. Analysis of the in vitro effects of di-(2-ethylhexyl) phthalate exposure on human uterine leiomyoma cells. Exp. Ther. Med. 2018, 15, 4972–4978. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, T.; Yin, P.; Zuberi, A.; Kujawa, S.; Coon, J.S.; Björvang, R.D.; Damdimopoulou, P.; Pacyga, D.C.; Strakovsky, R.S.; Flaws, J.A.; et al. Mono-(2-ethyl-5-hydroxyhexyl) phthalate promotes uterine leiomyoma cell survival through tryptophan-kynurenine-AHR pathway activation. Proc. Natl. Acad. Sci. USA 2022, 119, e2208886119. [Google Scholar] [CrossRef] [PubMed]
- Bidgoli, S.A.; Khorasani, H.; Keihan, H.; Sadeghipour, A.; Mehdizadeh, A. Role of endocrine disrupting chemicals in the occurrence of benign uterine leiomyomata: Special emphasis on AhR tissue levels. Asian Pac. J. Cancer Prev. 2012, 13, 5445–5450. [Google Scholar] [CrossRef]
- Dos Anjos, L.G.; de Almeida, B.C.; Baracat, E.C.; Al-Hendy, A.; Yang, Q.; Carvalho, K.C. Gene Expression Profile of Uterine Leiomyoma from Women Exposed to Different Air Pollution Levels in Metropolitan Cities of Sao Paulo, Brazil. Int. J. Mol. Sci. 2023, 24, 2431. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.C.; Li, W.-F.; Liao, P.-C.; Sun, C.-W.; Tsai, E.-M.; Wang, S.-L. Risk for estrogen-dependent diseases in relation to phthalate exposure and polymorphisms of CYP17A1 and estrogen receptor genes. Environ. Sci. Pollut. Res. Int. 2014, 21, 13964–13973. [Google Scholar] [CrossRef]
- Kim, J.L.; Kim, Y.-H.; Kang, M.-K.; Gong, J.-H.; Han, S.-J.; Kang, Y.-H. Antiosteoclastic activity of milk thistle extract after ovariectomy to suppress estrogen deficiency-induced osteoporosis. Biomed. Res. Int. 2013, 2013, 919374. [Google Scholar] [CrossRef]
- Pfingstgraf, I.O.; Taulescu, M.; Pop, R.M.; Orăsan, R.; Vlase, L.; Uifalean, A.; Todea, D.; Alexescu, T.; Toma, C.; Pârvu, A.E. Protective Effects of Taraxacum officinale L. (Dandelion) Root Extract in Experimental Acute on Chronic Liver Failure. Antioxidants 2021, 10, 504. [Google Scholar] [CrossRef]
- Wise, L.A. Study of Environment Lifestyle and Fibroids (SELF): Advancing the Field of Fibroid Epidemiology. J. Womens Health 2015, 24, 862–864. [Google Scholar] [CrossRef]
- Fibroid, H. Here Are the Best and Worst Foods for Fibroids. 2022. Available online: https://houstonfibroids.com/posts/diet-and-exercise/food-and-drinks-to-avoid-if-you-have-uterine-fibroids/ (accessed on 22 September 2023).
- Tinelli, A.; Vinciguerra, M.; Malvasi, A.; Andjić, M.; Babović, I.; Sparić, R. Uterine Fibroids and Diet. Int. J. Environ. Res. Public Health 2021, 18, 1066. [Google Scholar] [CrossRef]
- Parazzini, F.; Di Martino, M.; Candiani, M.; Viganò, P. Dietary components and uterine leiomyomas: A review of published data. Nutr. Cancer 2015, 67, 569–579. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, Y.; Lu, Q.; Ren, M. Vegetarian diet and reduced uterine fibroids risk: A case-control study in Nanjing, China. J. Obstet. Gynaecol. Res. 2016, 42, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.P.; Garzia, N.A.; Cushing-Haugen, K.; Terry, K.L.; Chiu, Y.-H.; Sandoval-Insausti, H.; Chavarro, J.E.; Missmer, S.A.; Harris, H.R. Fruit and vegetable consumption, pesticide residue intake from consumption of fruits and vegetables, and risk of uterine fibroids. F&S Sci. 2023, 4, 90–99. [Google Scholar]
- Islam, M.S.; Castellucci, C.; Fiorini, R.; Greco, S.; Gagliardi, R.; Zannotti, A.; Giannubilo, S.R.; Ciavattini, A.; Frega, N.G.; Pacetti, D.; et al. Omega-3 fatty acids modulate the lipid profile, membrane architecture, and gene expression of leiomyoma cells. J. Cell Physiol. 2018, 233, 7143–7156. [Google Scholar] [CrossRef] [PubMed]
- Lambertino, A.; Turyk, M.; Anderson, H.; Freels, S.; Persky, V. Uterine leiomyomata in a cohort of Great Lakes sport fish consumers. Environ. Res. 2011, 111, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Chiaffarino, F.; Parazzini, F.; La Vecchia, C.; Chatenoud, L.; Di Cintio, E.; Marsico, S. Diet and uterine myomas. Obstet. Gynecol. 1999, 94, 395–398. [Google Scholar]
- Lucero, J.; Harlow, B.L.; Barbieri, R.L.; Sluss, P.; Cramer, D.W. Early follicular phase hormone levels in relation to patterns of alcohol, tobacco, and coffee use. Fertil. Steril. 2001, 76, 723–729. [Google Scholar] [CrossRef]
- Leonard, T.K.; Watson, R.R.; Mohs, M.E. The effects of caffeine on various body systems: A review. J. Am. Diet Assoc. 1987, 87, 1048–1053. [Google Scholar] [CrossRef]
- Nagata, C.; Nakamura, K.; Oba, S.; Hayashi, M.; Takeda, N.; Yasuda, K. Association of intakes of fat, dietary fibre, soya isoflavones and alcohol with uterine fibroids in Japanese women. Br. J. Nutr. 2009, 101, 1427–1431. [Google Scholar] [CrossRef]
- Takala, H.; Yang, Q.; El Razek, A.M.A.; Ali, M.; Al-Hendy, A. Alcohol Consumption and Risk of Uterine Fibroids. Curr. Mol. Med. 2020, 20, 247–258. [Google Scholar] [CrossRef]
- Kim, S.; Han, K.; Choi, S.-Y.; Yang, S.Y.; Choi, S.H.; Yim, J.Y.; Kim, J.J.; Kim, M.-J. Alcohol consumption and the risk of new-onset uterine leiomyomas: A nationwide population-based study in 2.5 million Korean women aged 20 to 39 years. Am. J. Obstet. Gynecol. 2023, 229, e1–e45. [Google Scholar] [CrossRef]
- Ejaz, A.; Wu, D.; Kwan, P.; Meydani, M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J. Nutr. 2009, 139, 919–925. [Google Scholar] [CrossRef]
- Malik, M.; Britten, J.L.; DeAngelis, A.; Sitler, C.; Moran, S.; Roura-Monllor, J.A.; Driggers, P.; Catherino, W.H. Curcumin inhibits human leiomyoma xenograft tumor growth and induces dissolution of the extracellular matrix. F&S Sci. 2023, 4, 74–89. [Google Scholar]
- Ciebiera, M.; Ali, M.; Prince, L.; Jackson-Bey, T.; Atabiekov, I.; Zgliczyński, S.; Al-Hendy, A. The Evolving Role of Natural Compounds in the Medical Treatment of Uterine Fibroids. J. Clin. Med. 2020, 9, 1479. [Google Scholar] [CrossRef] [PubMed]
- Touré, A.; Xueming, X. Flaxseed Lignans: Source, Biosynthesis, Metabolism, Antioxidant Activity, Bio-Active Components, and Health Benefits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Ganbold, M.; Shimamoto, Y.; Ferdousi, F.; Tominaga, K.; Isoda, H. Antifibrotic effect of methylated quercetin derivatives on TGFβ-induced hepatic stellate cells. Biochem. Biophys. Rep. 2019, 20, 100678. [Google Scholar] [CrossRef]
- Ho, Y.; Yang, Y.-C.S.; Chin, Y.-T.; Chou, S.-Y.; Chen, Y.-R.; Shih, Y.-J.; Whang-Peng, J.; Changou, C.A.; Liu, H.-L.; Lin, S.-J.; et al. Resveratrol inhibits human leiomyoma cell proliferation via crosstalk between integrin αvβ3 and IGF-1R. Food Chem. Toxicol. 2018, 120, 346–355. [Google Scholar] [CrossRef]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.; Rezaei-Tavirani, M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef]
- Wu, H.-L.; Chuang, T.-Y.; Al-Hendy, A.; Diamond, M.P.; Azziz, R.; Chen, Y.-H. Berberine inhibits the proliferation of human uterine leiomyoma cells. Fertil. Steril. 2015, 103, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.-Y.; Min, J.; Wu, H.-L.; McCrary, C.; Layman, L.C.; Diamond, M.P.; Azziz, R.; Al-Hendy, A.; Chen, Y.-H. Berberine Inhibits Uterine Leiomyoma Cell Proliferation via Downregulation of Cyclooxygenase 2 and Pituitary Tumor-Transforming Gene 1. Reprod. Sci. 2017, 24, 1005–1013. [Google Scholar] [CrossRef]
- Wasternack, C. Jasmonates: An Update on Biosynthesis, Signal Transduction and Action in Plant Stress Response, Growth and Development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [PubMed]
- Filomeno, M.; Bosetti, C.; Bidoli, E.; Levi, F.; Serraino, D.; Montella, M.; La Vecchia, C.; Tavani, A. Mediterranean diet and risk of endometrial cancer: A pooled analysis of three Italian case-control studies. Br. J. Cancer 2015, 112, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S. U Current results on the biological and pharmacological activities of Indole-3-carbinol. Excli. J. 2018, 17, 181–185. [Google Scholar]
- Cho, S.; Kim, S.; Jin, Z.; Yang, H.; Han, D.; Baek, N.-I.; Jo, J.; Cho, C.-W.; Park, J.-H.; Shimizu, M.; et al. Isoliquiritigenin, a chalcone compound, is a positive allosteric modulator of GABAA receptors and shows hypnotic effects. Biochem. Biophys. Res. Commun. 2011, 413, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-H.; Kung, H.-L.; Chen, H.-Y.; Huang, K.-C.; Hsia, S.-M. Isoliquiritigenin Suppresses E2-Induced Uterine Leiomyoma Growth through the Modulation of Cell Death Program and the Repression of ECM Accumulation. Cancers 2019, 11, 1131. [Google Scholar] [CrossRef]
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling Anthocyanin Bioavailability for Human Health. Annu. Rev. Food Sci. Technol. 2016, 7, 375–393. [Google Scholar] [CrossRef]
- Lee, A.W.; Eata, V. Association of environmental phenols with endometriosis and uterine leiomyoma: An analysis of NHANES, 2003–2006. Reprod. Toxicol. 2022, 113, 30–34. [Google Scholar] [CrossRef]
- Chen, N.-N.; Han, M.; Yang, H.; Yang, G.-Y.; Wang, Y.-Y.; Wu, X.-K.; Liu, J.-P. Chinese herbal medicine Guizhi Fuling Formula for treatment of uterine fibroids: A systematic review of randomised clinical trials. BMC Complement Altern. Med. 2014, 14, 2. [Google Scholar] [CrossRef]
- Castro, L.; Gao, X.; Moore, A.B.; Yu, L.; Di, X.; Kissling, G.E.; Dixon, D. A High Concentration of Genistein Induces Cell Death in Human Uterine Leiomyoma Cells by Autophagy. Expert. Opin. Environ. Biol. 2016, 5 (Suppl. S1). [Google Scholar] [CrossRef]
- Tuzcu, M.; Sahin, N.; Ozercan, I.; Seren, S.; Sahin, K.; Kucuk, O. The effects of selenium supplementation on the spontaneously occurring fibroid tumors of oviduct, 8-hydroxy-2’-deoxyguanosine levels, and heat shock protein 70 response in Japanese quail. Nutr. Cancer 2010, 62, 495–500. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vafaei, S.; Ciebiera, M.; Omran, M.M.; Ghasroldasht, M.M.; Yang, Q.; Leake, T.; Wolfe, R.; Ali, M.; Al-Hendy, A. Evidence-Based Approach for Secondary Prevention of Uterine Fibroids (The ESCAPE Approach). Int. J. Mol. Sci. 2023, 24, 15972. https://doi.org/10.3390/ijms242115972
Vafaei S, Ciebiera M, Omran MM, Ghasroldasht MM, Yang Q, Leake T, Wolfe R, Ali M, Al-Hendy A. Evidence-Based Approach for Secondary Prevention of Uterine Fibroids (The ESCAPE Approach). International Journal of Molecular Sciences. 2023; 24(21):15972. https://doi.org/10.3390/ijms242115972
Chicago/Turabian StyleVafaei, Somayeh, Michał Ciebiera, Mervat M. Omran, Mohammad Mousaei Ghasroldasht, Qiwei Yang, Tanya Leake, Rochelle Wolfe, Mohamed Ali, and Ayman Al-Hendy. 2023. "Evidence-Based Approach for Secondary Prevention of Uterine Fibroids (The ESCAPE Approach)" International Journal of Molecular Sciences 24, no. 21: 15972. https://doi.org/10.3390/ijms242115972
APA StyleVafaei, S., Ciebiera, M., Omran, M. M., Ghasroldasht, M. M., Yang, Q., Leake, T., Wolfe, R., Ali, M., & Al-Hendy, A. (2023). Evidence-Based Approach for Secondary Prevention of Uterine Fibroids (The ESCAPE Approach). International Journal of Molecular Sciences, 24(21), 15972. https://doi.org/10.3390/ijms242115972