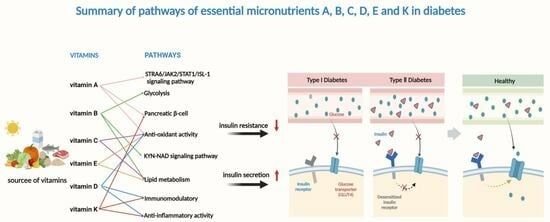
Research Progress on the Relationship between Vitamins and Diabetes: Systematic Review
Abstract
:1. Introduction
2. Methods
Data Sources and Data Extraction
3. Results and Discussion
3.1. Vitamin A
3.1.1. Biological Functions
3.1.2. Vitamin A and Diabetes
3.2. B Vitamins
3.2.1. Biological Functions
3.2.2. B Vitamins and Diabetes
3.3. Antioxidant Vitamins C and E
3.3.1. Biological Functions
3.3.2. Vitamins C and E and Diabetes
3.4. Vitamin D
3.4.1. Biological Functions
3.4.2. Vitamin D and Diabetes
3.5. Vitamin K
3.5.1. Biological Functions
3.5.2. Vitamin K and Diabetes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Demir, S.; Nawroth, P.P.; Herzig, S.; Ekim Üstünel, B. Emerging Targets in Type 2 Diabetes and Diabetic Complications. Adv. Sci. (Weinh) 2021, 8, e2100275. [Google Scholar] [CrossRef] [PubMed]
- Adeghate, S.E. Chronic Complications of Diabetes Mellitus: A Mini Review. Curr. Diabetes Rev. 2017, 13, 3–10. [Google Scholar]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45, S17–S38. [Google Scholar] [CrossRef]
- Kerru, N.; Singh-Pillay, A.; Awolade, P.; Singh, P. Current Anti-Diabetic Agents and Their Molecular Targets: A Review. Eur. J. Med. Chem. Chim. Ther. 2018, 152, 436–488. [Google Scholar]
- Kayaniyil, S.; Vieth, R.; Retnakaran, R.; Knight, J.A.; Qi, Y.; Gerstein, H.C.; Perkins, B.A.; Harris, S.B.; Zinman, B.; Hanley, A.J. Association of Vitamin D with Insulin Resistance and β-Cell Dysfunction in Subjects at Risk for Type 2 Diabetes. Diabetes Care 2010, 33, 1379–1381. [Google Scholar] [CrossRef]
- Palomer, X.; Gonzalez-Clemente, J.M.; Blanco-Vaca, F.; Mauricio, D. Role of Vitamin D in the Pathogenesis of Type 2 Diabetes Mellitus. Diabetes Obes. Metab. 2008, 10, 185–197. [Google Scholar] [CrossRef]
- Rösen, P.; Nawroth, P.P.; King, G.; Möller, W.; Tritschler, H.J.; Packer, L. The Role of Oxidative Stress in the Onset and Progression of Diabetes and Its Complications: A Summary of a Congress Series Sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab. Res. Rev. 2010, 17, 189–212. [Google Scholar] [CrossRef]
- Blaner, W.S. Vitamin A Signaling and Homeostasis in Obesity, Diabetes, and Metabolic Disorders. Pharmacol. Ther. 2019, 197, 153–178. [Google Scholar]
- Prentice, R.L.; Pettinger, M.; Neuhouser, M.L.; Tinker, L.F.; Huang, Y.; Zheng, C.; Manson, J.E.; Mossavar-Rahmani, Y.; Anderson, G.L.; Lampe, J.W. Application of Blood Concentration Biomarkers in Nutritional Epidemiology: Example of Carotenoid and Tocopherol Intake in Relation to Chronic Disease Risk. Am. J. Clin. Nutr. 2019, 109, 1189–1196. [Google Scholar] [CrossRef]
- Sluijs, I.; Cadier, E.; Beulens, J.W.; van der A., D.L.; Spijkerman, A.M.; van der Schouw, Y.T. Dietary intake of carotenoids and risk of type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 376–381. [Google Scholar] [PubMed]
- Kataja-Tuomola, M.; Sundell, J.R.; Männistö, S.; Virtanen, M.J.; Kontto, J.; Albanes, D.; Virtamo, J. Effect of Alpha-Tocopherol and Beta-Carotene Supplementation on the Incidence of Type 2 Diabetes. Diabetologia 2008, 51, 47–53. [Google Scholar] [CrossRef]
- de Oliveira Otto, M.C.; Alonso, A.; Lee, D.H.; Delclos, G.L.; Bertoni, A.G.; Jiang, R.; Lima, J.A.; Symanski, E.; Jacobs, D.R., Jr.; Nettleton, J.A. Dietary Intakes of Zinc and Heme Iron from Red Meat, But Not from Other Sources, Are Associated with Greater Risk of Metabolic Syndrome and Cardiovascular Disease. J. Nutr. 2012, 142, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Eshak, E.S.; Iso, H.; Muraki, I.; Tamakoshi, A. Fat-Soluble Vitamins from Diet in Relation to Risk of Type 2 Diabetes Mellitus in Japanese Population. Br. J. Nutr. 2019, 121, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; He, J.; Liu, Z.; Wu, S.; Chen, P.; Li, K.; Fang, A. Dietary Total Vitamin A, β-Carotene, and Retinol Intake and the Risk of Diabetes in Chinese Adults with Plant-Based Diets. J. Clin. Endocrinol. Metab. 2022, 107, e4106–e4114. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, J.; Sun, B.; Xu, W.; Sun, Z. Vitamin A Deficiency Causes Islet Dysfunction by Inducing Islet Stellate Cell Activation via Cellular Retinol Binding Protein 1. Int. J. Biol. Sci. 2020, 16, 947–956. [Google Scholar] [CrossRef]
- Trasino, S.E.; Benoit, Y.D.; Gudas, L.J. Vitamin A Deficiency Causes Hyperglycemia and Loss of Pancreatic β-Cell Mass. J. Biol. Chem. 2015, 290, 1456–1473. [Google Scholar]
- Fan, J.; Yin, S.; Lin, D.; Liu, Y.; Xia, M. Association of Serum Retinol-Binding Protein 4 Levels and the Risk of Incident Type 2 Diabetes in Subjects with Prediabetes. Diabetes Care 2019, 42, 1574–1581. [Google Scholar]
- Huang, R.; Bai, X.; Li, X.; Wang, X.; Zhao, L. Retinol-Binding Protein 4 Activates STRA6 Provoking Pancreatic β Cell Dysfunction in Type 2 Diabetes. Diabetes 2020, 70, db191241. [Google Scholar]
- Polizzi, F.C.; Andican, G.; Çetin, E.; Civelek, S.; Yumuk, V.; Burçak, G. Increased DNA-Glycation in Type 2 Diabetic Patients: The Effect of Thiamine and Pyridoxine Therapy. Exp. Clin. Endocrinol. Diabetes 2012, 120, 329–334. [Google Scholar] [CrossRef]
- Waheed, P.; Naveed, A.K.; Ahmed, T. Thiamine Deficiency and Its Correlation with Dyslipidaemia in Diabetics with Microalbuminuria. J. Pak. Med. Assoc. 2013, 63, 340–345. [Google Scholar] [PubMed]
- Takeuchi, F.; Tsubouchi, R.; Izuta, S.; Shibata, Y. Kynurenine metabolism and Xanthurenic Acid Formation in Vitamin B6-Deficient Rat after Tryptophan Injection. J. Nutr. Sci. Vitaminol. 1989, 35, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Jove, M.; Ortega, F.; Xifra, G.; Ricart, W.; Obis, È.; Pamplona, R.; Portero-Otin, M.; Fernández-Real, J.M. Metabolomics Uncovers the Role of Adipose Tissue PDXK in Adipogenesis and Systemic Insulin Sensitivity. Diabetologia 2016, 59, 822–832. [Google Scholar] [CrossRef]
- Yanaka, N.; Kanda, M.; Toya, K.; Suehiro, H.; Kato, N. Vitamin B6 Regulates mRNA Expression of Peroxisome Proliferator-Activated Receptor-γ Target Genes. Exp. Ther. Med. 2011, 2, 419–424. [Google Scholar] [CrossRef]
- Liu, Z.; Li, P.; Zhao, Z.H.; Zhang, Y.; Ma, Z.M.; Wang, S.X. Vitamin B6 Prevents Endothelial Dysfunction, Insulin Resistance, and Hepatic Lipid Accumulation in Apoe(−/−) Mice Fed with High-Fat Diet. J. Diabetes Res. 2016, 2016, 1748065. [Google Scholar] [CrossRef] [PubMed]
- Tareke, A.A.; Hadgu, A.A. The Effect of Vitamin C Supplementation on Lipid Profile of Type 2 Diabetic Patients: A Systematic Review and Meta-Analysis of Clinical Trials. Diabetol. Metab. Syndr. 2021, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Keske, M.A.; Wadley, G.D. Effects of Vitamin C Supplementation on Glycemic Control and Cardiovascular Risk Factors in People with Type 2 Diabetes: A GRADE-Assessed Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Care 2021, 44, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Oh, Y.K.; Lee, J.; Kim, E. Could Nutrient Supplements Provide Additional Glycemic Control in Diabetes Management? A Systematic Review and Meta-Analysis of Randomized Controlled Trials of as an Add-on Nutritional Supplementation Therapy. Arch. Pharm. Res. 2022, 45, 185–204. [Google Scholar] [CrossRef]
- El-Aal, A.A.; El-Ghffar, E.A.A.; Ghali, A.A.; Zughbur, M.R.; Sirdah, M.M. The Effect of Vitamin C and/or E Supplementations on Type 2 Diabetic Adult Males under Metformin Treatment: A Single-Blinded Randomized Controlled Clinical Trial. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 483–489. [Google Scholar] [CrossRef]
- Siavash, M.; Amini, M. Vitamin C May Have Similar Beneficial Effects to Gemfibrozil on Serum High-Density Lipoprotein-Cholesterol in Type 2 Diabetic Patients. J. Res. Pharm. Pract. 2014, 3, 77–82. [Google Scholar] [CrossRef]
- Dakhale, G.N.; Chaudhari, H.V.; Shrivastava, M. Supplementation of Vitamin C Reduces Blood Glucose and Improves Glycosylated Hemoglobin in Type 2 Diabetes Mellitus: A Randomized, Double-Blind Study. Adv. Pharmacol. Sci. 2011, 2011, 195271. [Google Scholar] [CrossRef] [PubMed]
- Paolisso, G.; Balbi, V.; Volpe, C.; Varricchio, G.; Gambardella, A.; Saccomanno, F.; Ammendola, S.; Varricchio, M.; D’Onofrio, F. Metabolic Benefits Deriving from Chronic Vitamin C Supplementation in Aged Non-Insulin Dependent Diabetics. J. Am. Coll. Nutr. 1995, 14, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.J.; Jeong, Y.C.; Choi, J.H. Effects of Vitamin E on Phospholipase A2 Activity and Oxidative Damage to the Liver in Streptozotocin-Induced Diabetic Rats. Ann. Nutr. Metab. 2005, 49, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Vinayagamoorthi, R.; Bobby, Z.; Sridhar, M.G. Antioxidants Preserve Redox Balance and Inhibit c-Jun-N-Terminal Kinase Pathway While Improving Insulin Signaling in Fat-Fed Rats: Evidence for the Role of Oxidative Stress on IRS-1 Serine Phosphorylation and Insulin Resistance. J. Endocrinol. 2008, 197, 287–296. [Google Scholar] [CrossRef]
- Abdel-Wahab, Y.H.; O’Harte, F.P.; Mooney, M.H.; Barnett, C.R.; Flatt, P.R. Vitamin C Supplementation Decreases Insulin Glycation and Improves Glucose Homeostasis in Obese Hyperglycemic (ob/ob) Mice. Metabolism 2002, 51, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Leonard, S.; Traber, M.G.; Jialal, I. Gamma-Tocopherol Supplementation Alone and in Combination with alpha-Tocopherol Alters Biomarkers of Oxidative Stress and Inflammation in Subjects with Metabolic Syndrome. Free. Radic. Biol. Med. 2008, 44, 1203–1208. [Google Scholar] [CrossRef]
- Ashor, A.W.; Werner, A.D.; Lara, J.; Willis, N.D.; Mathers, J.C.; Siervo, M. Effects of Vitamin C Supplementation on Glycaemic Control: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Eur. J. Clin. Nutr. 2017, 71, 1371–1380. [Google Scholar] [CrossRef]
- Kalliora, M.I.; Vazeou, A.; Delis, D.; Bozas, E.; Thymelli, I.; Bartsocas, C.S. Seasonal Variation of Type 1 Diabetes Mellitus Diagnosis in Greek Children. Hormones 2011, 10, 67–71. [Google Scholar] [CrossRef]
- Svensson, J.; Lyngaae-Jørgensen, A.; Carstensen, B.; Simonsen, L.B.; Mortensen, H.B. Long-Term Trends in the Incidence of Type 1 Diabetes in Denmark: The Seasonal Variation Changes over Time. Pediatr. Diabetes 2009, 10, 248–254. [Google Scholar] [CrossRef]
- Wu, J.; Shao, B.; Xin, X.; Luo, W.; Mo, M.; Jiang, W.; Si, S.; Wang, S.; Shen, Y.; Yu, Y. Association of Vitamin D Pathway Gene Polymorphisms with Vitamin D Level during Pregnancy Was Modified by Season and Vitamin D Supplement. Clin. Nutr. 2021, 40, 3650–3660. [Google Scholar] [CrossRef]
- Wei, Z.; Yoshihara, E.; He, N.; Hah, N.; Fan, W.; Pinto, A.F.M.; Huddy, T.; Wang, Y.; Ross, B.; Estepa, G.; et al. Vitamin D Switches BAF Complexes to Protect β Cells. Cell 2018, 173, 1135–1149.e15. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.K.B.; Sulis, P.M.; Cavalari, F.C.; Padilla, D.P.R.; Aragón, M.; Gaspar, J.M.; Silva, F. 1α,25-(OH)(2) Vitamin D(3) Prevents Insulin Resistance and Regulates Coordinated Exocytosis and Insulin Secretion. J. Nutr. Biochem. 2022, 99, 108864. [Google Scholar] [CrossRef] [PubMed]
- Panjiyar, R.P.; Dayal, D.; Attri, S.V.; Sachdeva, N.; Sharma, R.; Bhalla, A.K. Sustained Serum 25-Hydroxyvitamin D Concentrations for One Year with Cholecalciferol Supplementation Improves Glycaemic Control and Slows the Decline of Residual β Cell Function in Children with Type 1 Diabetes. Pediatr. Endocrinol. Diabetes Metab. 2018, 2018, 111–117. [Google Scholar] [CrossRef]
- Sharma, S.; Biswal, N.; Bethou, A.; Rajappa, M.; Kumar, S.; Vinayagam, V. Does Vitamin D Supplementation Improve Glycaemic Control in Children with Type 1 Diabetes Mellitus?—A Randomized Controlled Trial. J. Clin. Diagn. Res. 2017, 11, sc15–sc17. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Dawson-Hughes, B.; Sheehan, P.; Ware, J.H.; Knowler, W.C.; Aroda, V.R.; Brodsky, I.; Ceglia, L.; Chadha, C.; Chatterjee, R.; et al. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 520–530. [Google Scholar] [CrossRef]
- Norman, A.W.; Frankel, J.B.; Heldt, A.M.; Grodsky, G.M. Vitamin D Deficiency Inhibits Pancreatic Secretion of Insulin. Science 1980, 209, 823–825. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, P.; Weisnagel, S.J.; Caron, A.Z.; Julien, A.S.; Morisset, A.S.; Carreau, A.M.; Poirier, J.; Tchernof, A.; Robitaille, J.; Bergeron, J.; et al. Effects of 6-Month Vitamin D Supplementation on Insulin Sensitivity and Secretion: A Randomised, Placebo-Controlled Trial. Eur. J. Endocrinol. 2019, 181, 287–299. [Google Scholar] [CrossRef]
- Juanola-Falgarona, M.; Salas-Salvadó, J.; Estruch, R.; Portillo, M.P.; Casas, R.; Miranda, J.; Martínez-González, M.A.; Bulló, M. Association between Dietary Phylloquinone Intake and Peripheral Metabolic Risk Markers Related to Insulin Resistance and Diabetes in Elderly Subjects at High Cardiovascular Risk. Cardiovasc. Diabetol. 2013, 12, 7. [Google Scholar] [CrossRef]
- Yoshida, M.; Booth, S.L.; Meigs, J.B.; Saltzman, E.; Jacques, P.F. Phylloquinone Intake, Insulin Sensitivity, and Glycemic Status in Men and Women. Am. J. Clin. Nutr. 2008, 88, 210–215. [Google Scholar] [CrossRef]
- Beulens, J.W.; van der A, A.D.; Grobbee, D.E.; Sluijs, I.; Spijkerman, A.M.; van der Schouw, Y.T. Dietary Phylloquinone and Menaquinones Intakes and Risk of Type 2 Diabetes. Diabetes Care 2010, 33, 1699–1705. [Google Scholar] [CrossRef]
- Sakamoto, N.; Nishiike, T.; Iguchi, H.; Sakamoto, K. Possible Effects of One Week Vitamin K (Menaquinone-4) Tablets Intake on Glucose Tolerance in Healthy Young Male Volunteers with Different Descarboxy Prothrombin Levels. Clin. Nutr. 2000, 19, 259–263. [Google Scholar] [CrossRef]
- Choi, H.J.; Yu, J.; Choi, H.; An, J.H.; Kim, S.W.; Park, K.S.; Jang, H.C.; Kim, S.Y.; Shin, C.S. Vitamin K2 Supplementation Improves Insulin Sensitivity via Osteocalcin Metabolism: A Placebo-Controlled Trial. Diabetes Care 2011, 34, e147. [Google Scholar] [CrossRef] [PubMed]
- Zwakenberg, S.R.; Remmelzwaal, S.; Beulens, J.W.J.; Booth, S.L.; Burgess, S.; Dashti, H.S.; Imamura, F.; Feskens, E.J.M.; van der Schouw, Y.T.; Sluijs, I. Circulating Phylloquinone Concentrations and Risk of Type 2 Diabetes: A Mendelian Randomization Study. Diabetes 2019, 68, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Ferron, M.; Hinoi, E.; Karsenty, G.; Ducy, P. Osteocalcin Differentially Regulates Beta Cell and Adipocyte Gene Expression and Affects the Development of Metabolic Diseases in Wild-Type Mice. Proc. Natl. Acad. Sci. USA 2008, 105, 5266–5270. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, Y.; Shirakawa, H.; Miura, A.; Giriwono, P.E.; Sato, S.; Ohashi, A.; Iribe, M.; Goto, T.; Komai, M. Vitamin K Suppresses the Lipopolysaccharide-Induced Expression of Inflammatory Cytokines in Cultured Macrophage-Like Cells via the Inhibition of the Activation of Nuclear Factor κB through the Repression of IKKα/β Phosphorylation. J. Nutr. Biochem. 2010, 21, 1120–1126. [Google Scholar] [CrossRef]
- Sogabe, N.; Maruyama, R.; Baba, O.; Hosoi, T.; Goseki-Sone, M. Effects of Long-Term Vitamin K(1) (Phylloquinone) or Vitamin K(2) (Menaquinone-4) Supplementation on Body Composition and Serum Parameters in Rats. Bone 2011, 48, 1036–1042. [Google Scholar] [CrossRef]
- Saeterdal, I.; Mora, J.O.; De-Regil, L.M. Fortification of Staple Foods with Vitamin A for Preventing Vitamin A Deficiency; The Cochrane Library: Hoboken, NJ, USA, 2012. [Google Scholar]
- Wei, C.; Guoxun, C. The Roles of Vitamin A in the Regulation of Carbohydrate, Lipid, and Protein Metabolism. J. Clin. Med. 2014, 3, 453–479. [Google Scholar]
- Saeed, A.; Dullaart, R.P.F.; Schreuder, T.C.M.A.; Blokzijl, H. Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2018, 10, 29. [Google Scholar] [CrossRef]
- Kennedy, D.O. B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef]
- Hanna, M.; Jaqua, E.; Nguyen, V.; Clay, J. B Vitamins: Functions and Uses in Medicine. Perm. J. 2022, 26, 89–97. [Google Scholar] [CrossRef]
- Jansen, B.C.P.; Donath, W.F. Geneeskundig Tijdschrift Voor Nederlandsch-Indie. Nutr. Rev. 1982, 40, 53–54. [Google Scholar] [CrossRef]
- Janes, R.G.; Brady, J.M. Thiamine Deficiency in Adult Normal and Diabetic Rats as Studied under Paired-Feeding Conditions. Fed. Proc. 1947, 6 Pt 2, 136. [Google Scholar] [PubMed]
- Beltramo, E.; Berrone, E.; Tarallo, S.; Porta, M. Effects of Thiamine and Benfotiamine on Intracellular Glucose Metabolism and Relevance in the Prevention of Diabetic Complications. Acta Diabetol. 2008, 45, 131–141. [Google Scholar] [CrossRef]
- Mascolo, E.; Vernì, F. Vitamin B6 and Diabetes: Relationship and Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 3669. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G. Insulin Resistance and Dysregulation of Tryptophan-Kynurenine and Kynurenine-Nicotinamide Adenine Dinucleotide Metabolic Pathways. Mol. Neurobiol. 2013, 48, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Ala, O.A.; Akintunde, A.A.; Ikem, R.T.; Kolawole, B.A.; Ala, O.O.; Adedeji, T.A. Association between Insulin Resistance and Total Plasma Homocysteine Levels in Type 2 Diabetes Mellitus Patients in South West Nigeria. Diabetes Metab. Syndr. 2017, 11 (Suppl. S2), s803–s809. [Google Scholar] [CrossRef]
- Azzini, E.; Ruggeri, S.; Polito, A. Homocysteine: Its Possible Emerging Role in At-Risk Population Groups. Int. J. Mol. Sci. 2020, 21, 1421. [Google Scholar] [CrossRef]
- Pratama, S.; Lauren, B.C.; Wisnu, W. The Efficacy of Vitamin B(12) Supplementation for Treating Vitamin B(12) Deficiency and Peripheral Neuropathy in Metformin-Treated Type 2 Diabetes Mellitus Patients: A Systematic Review. Diabetes Metab. Syndr. 2022, 16, 102634. [Google Scholar] [CrossRef]
- Liu, C.; Zhong, C.; Chen, R.; Zhou, X.; Wu, J.; Han, J.; Li, X.; Zhang, Y.; Gao, Q.; Xiao, M.; et al. Higher Dietary Vitamin C Intake Is Associated with a Lower Risk of Gestational Diabetes Mellitus: A Longitudinal Cohort Study. Clin. Nutr. 2020, 39, 198–203. [Google Scholar] [CrossRef]
- Vasudevan, S.; Hirsch, I.B. Interference of Intravenous Vitamin C with Blood Glucose Testing. Diabetes Care 2014, 37, e93–e94. [Google Scholar] [CrossRef]
- Yan, M.K.; Khalil, H. Vitamin Supplements in Type 2 Diabetes Mellitus Management: A Review. Diabetes Metab. Syndr. 2017, 11 (Suppl. S2), s589–s595. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Rasmussen, B.; van Loon, L.J.C.; Salmon, J.; Wadley, G.D. Ascorbic Acid Supplementation Improves Postprandial Glycaemic Control and Blood Pressure in Individuals with Type 2 Diabetes: Findings of a Randomized Cross-Over Trial. Diabetes Obes. Metab. 2019, 21, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Rueangdetnarong, H.; Sekararithi, R.; Jaiwongkam, T.; Kumfu, S.; Chattipakorn, N.; Tongsong, T.; Jatavan, P. Comparisons of the Oxidative Stress Biomarkers Levels in Gestational Diabetes Mellitus (GDM) and Non-GDM among Thai Population: Cohort Study. Endocr. Connect. 2018, 7, 681–687. [Google Scholar] [CrossRef]
- Altomare, E.; Vendemiale, G.; Chicco, D.; Procacci, V.; Cirelli, F. Increased Lipid Peroxidation in Type 2 Poorly Controlled Diabetic Patients. Diabete Metab. 1992, 18, 264–271. [Google Scholar] [PubMed]
- Yao, M.; Xu, F.; Yao, Y.; Wang, H.; Ju, X.; Wang, L. Assessment of Novel Oligopeptides from Rapeseed Napin (Brassica napus) in Protecting HepG2 Cells from Insulin Resistance and Oxidative Stress. J. Agric. Food Chem. 2022, 70, 12418–12429. [Google Scholar] [CrossRef]
- Hurrle, S.; Hsu, W.H. The Etiology of Oxidative Stress in Insulin Resistance. Biomed. J. 2017, 40, 257–262. [Google Scholar] [CrossRef]
- Balbi, M.E.; Tonin, F.S.; Mendes, A.M.; Borba, H.H.; Wiens, A.; Fernandez-Llimos, F.; Pontarolo, R. Antioxidant Effects of Vitamins in Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Diabetol. Metab. Syndr. 2018, 10, 18. [Google Scholar] [CrossRef]
- Paolisso, G.; D’Amore, A.; Giugliano, D.; Ceriello, A.; Varricchio, M.; D’Onofrio, F. Pharmacologic Doses of Vitamin E Improve Insulin Action in Healthy Subjects and Non-Insulin-Dependent Diabetic Patients. Am. J. Clin. Nutr. 1993, 57, 650–656. [Google Scholar] [CrossRef]
- Paolisso, G.; D’Amore, A.; Balbi, V.; Volpe, C.; Galzerano, D.; Giugliano, D.; Sgambato, S.; Varricchio, M.; D’Onofrio, F. Plasma Vitamin C Affects Glucose Homeostasis in Healthy Subjects and in Non-Insulin-Dependent Diabetics. Am. J. Physiol. 1994, 266 Pt 1, E261–E268. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative Stress, Insulin Signaling, and Diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef]
- Bloch-Damti, A.; Bashan, N. Proposed Mechanisms for the Induction of Insulin Resistance by Oxidative Stress. Antioxid. Redox Signal. 2005, 7, 1553–1567. [Google Scholar] [CrossRef] [PubMed]
- Liebler, D.C.; Kaysen, K.L.; Kennedy, T.A. Redox Cycles of Vitamin E: Hydrolysis and Ascorbic acid Dependent Reduction of 8a-(Alkyldioxy)Tocopherones. Biochemistry 1989, 28, 9772–9777. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Hashimoto, T.; Tokumaru, S.; Iguchi, H.; Kojo, S. Interactions between Vitamin C and Vitamin E Are Observed in Tissues of Inherently Scorbutic Rats. J. Nutr. 1997, 127, 2060–2064. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Song, H. Antioxidant Vitamins Intake and the Risk of Coronary Heart Disease: Meta-Analysis of Cohort Studies. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 15, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Frydrych, L.M.; Fattahi, F.; He, K.; Ward, P.A.; Delano, M.J. Diabetes and Sepsis: Risk, Recurrence, and Ruination. Front. Endocrinol. 2017, 8, 271. [Google Scholar] [CrossRef]
- Hongsawong, N.; Chawprang, N.; Kittisakmontri, K.; Vittayananan, P.; Srisuwan, K.; Chartapisak, W. Vitamin C Deficiency and Impact of Vitamin C Administration among Pediatric Patients with Advanced Chronic Kidney Disease. Pediatr. Nephrol. 2021, 36, 397–408. [Google Scholar] [CrossRef]
- McLean, F.C.; Budy, A.M. Vitamin A, Vitamin D, Cartilage, Bones, and Teeth. Vitam. Horm. 1963, 21, 51–68. [Google Scholar] [CrossRef]
- Norman, A.W. The History of the Discovery of Vitamin D and Its Daughter Steroid Hormone. Ann. Nutr. Metab. 2012, 61, 199–206. [Google Scholar] [CrossRef]
- Holick, M.F.; Schnoes, H.K.; DeLuca, H.F. Identification of 1,25-Dihydroxycholecalciferol, a Form of Vitamin D3 Metabolically Active in the Intestine. Proc. Natl. Acad. Sci. USA 1971, 68, 803–804. [Google Scholar] [CrossRef]
- Quaresima, P.; Angeletti, M.; Luziatelli, D.; Luziatelli, S.; Venturella, R.; Di Carlo, C.; Bernardo, S. Pregnancy Associated Transient Osteoporosis of the Hip (PR-TOH): A Non-Obstetric Indication to Caesarean Section. A Case Report with Literature Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 262, 28–35. [Google Scholar] [CrossRef]
- Cândido, F.G.; Bressan, J. Vitamin D: Link between Osteoporosis, Obesity, and Diabetes? Int. J. Mol. Sci. 2014, 15, 6569–6591. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Bennett, D.A.; Millwood, I.Y.; Parish, S.; McCarthy, M.I.; Mahajan, A.; Lin, X.; Bragg, F.; Guo, Y.; Holmes, M.V.; et al. Association of Vitamin D with Risk of Type 2 Diabetes: A Mendelian Randomisation Study in European and Chinese Adults. PLoS Med. 2018, 15, e1002566. [Google Scholar] [CrossRef] [PubMed]
- Rihal, V.; Khan, H.; Kaur, A.; Singh, T.G. Vitamin D as Therapeutic Modulator in Cerebrovascular Diseases: A Mechanistic Perspectives. Crit. Rev. Food Sci. Nutr. 2022, 63, 7772–7794. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qin, S.; Zhang, S.; Lu, Y.; Shen, Q.; Cheng, L.; Zhong, R. Serum Vitamin D Concentration, Vitamin D-Related Polymorphisms, and Colorectal Cancer Risk. Int. J. Cancer 2023, 153, 278–289. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Farruggia, M.; Veronese, N.; Barbagallo, M. Vitamin D Sources, Metabolism, and Deficiency: Available Compounds and Guidelines for Its Treatment. Metabolites 2021, 11, 255. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Neu, A.; Kehrer, M.; Hub, R.; Ranke, M.B. Incidence of IDDM in German Children Aged 0–14 Years. A 6-Year Population-Based Study (1987–1993). Diabetes Care 1997, 20, 530–533. [Google Scholar] [CrossRef]
- Karvonen, M.; Jäntti, V.; Muntoni, S.; Stabilini, M.; Stabilini, L.; Muntoni, S.; Tuomilehto, J. Comparison of the Seasonal Pattern in the Clinical Onset of IDDM in Finland and Sardinia. Diabetes Care 1998, 21, 1101–1109. [Google Scholar] [CrossRef]
- Moltchanova, E.V.; Schreier, N.; Lammi, N.; Karvonen, M. Seasonal Variation of Diagnosis of Type 1 Diabetes Mellitus in Children Worldwide. Diabet. Med. 2009, 26, 673–678. [Google Scholar] [CrossRef]
- Bailey, R.; Cooper, J.D.; Zeitels, L.; Smyth, D.J.; Yang, J.H.; Walker, N.M.; Hyppönen, E.; Dunger, D.B.; Ramos-Lopez, E.; Badenhoop, K.; et al. Association of the Vitamin D Metabolism Gene CYP27B1 with Type 1 Diabetes. Diabetes 2007, 56, 2616–2621. [Google Scholar] [CrossRef]
- Cooper, J.D.; Smyth, D.J.; Walker, N.M.; Stevens, H.; Burren, O.S.; Wallace, C.; Greissl, C.; Ramos-Lopez, E.; Hyppönen, E.; Dunger, D.B.; et al. Inherited Variation in Vitamin D Genes is Associated with Predisposition to Autoimmune Disease Type 1 Diabetes. Diabetes 2011, 60, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Nejentsev, S.; Cooper, J.D.; Godfrey, L.; Howson, J.M.; Rance, H.; Nutland, S.; Walker, N.M.; Guja, C.; Ionescu-Tirgovişte, C.; Savage, D.A.; et al. Analysis of the Vitamin D Receptor Gene Sequence Variants in Type 1 Diabetes. Diabetes 2004, 53, 2709–2712. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Kawahara, T.; Jorde, R.; Dawson-Hughes, B.; Vickery, E.M.; Angellotti, E.; Nelson, J.; Trikalinos, T.A.; Balk, E.M. Vitamin D and Risk for Type 2 Diabetes in People with Prediabetes: A Systematic Review and Meta-Analysis of Individual Participant Data from 3 Randomized Clinical Trials. Ann. Intern. Med. 2023, 176, 355–363. [Google Scholar] [CrossRef]
- Van Belle, T.L.; Gysemans, C.; Mathieu, C. Vitamin D and diabetes: The Odd Couple. Trends Endocrinol. Metab. 2013, 24, 561–568. [Google Scholar] [CrossRef]
- Cade, C.; Norman, A.W. Rapid Normalization/Stimulation by 1,25-Dihydroxyvitamin D3 of Insulin Secretion and Glucose Tolerance in the Vitamin D-Deficient Rat. Endocrinology 1987, 120, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, N.; Brodsky, I.G.; Chatterjee, R.; Kim, S.H.; Pratley, R.E.; Staten, M.A.; Pittas, A.G. Effects of Vitamin D Supplementation on Insulin Sensitivity and Secretion in Prediabetes. J. Clin. Endocrinol. Metab. 2022, 107, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Provvedini, D.M.; Tsoukas, C.D.; Deftos, L.J.; Manolagas, S.C. 1,25-Dihydroxyvitamin D3 Receptors in Human Leukocytes. Science 1983, 221, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Provvedini, D.M.; Tsoukas, C.D.; Deftos, L.J.; Manolagas, S.C. 1 Alpha,25-Dihydroxyvitamin D3-Binding Macromolecules in Human B Lymphocytes: Effects on Immunoglobulin Production. J. Immunol. 1986, 136, 2734–2740. [Google Scholar] [CrossRef]
- Veldman, C.M.; Cantorna, M.T.; DeLuca, H.F. Expression of 1,25-Dihydroxyvitamin D(3) Receptor in the Immune System. Arch. Biochem. Biophys. 2000, 374, 334–338. [Google Scholar] [CrossRef]
- Wolden-Kirk, H.; Overbergh, L.; Christesen, H.T.; Brusgaard, K.; Mathieu, C. Vitamin D and Diabetes: Its Importance for Beta Cell and Immune Function. Mol. Cell Endocrinol. 2011, 347, 106–120. [Google Scholar] [CrossRef]
- Fusaro, M.; Gallieni, M.; Porta, C.; Nickolas, T.L.; Khairallah, P. Vitamin K Effects in Human Health: New Insights beyond Bone and Cardiovascular Health. J. Nephrol. 2020, 33, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.L.; Karl, J.P.; Oliverio, A.M.; Fu, X.; Soares, J.W.; Wolfe, B.E.; Hernandez, C.J.; Mason, J.B.; Booth, S.L. Dietary Vitamin K Is Remodeled by Gut Microbiota and Influences Community Composition. Gut Microbes 2021, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, L.; Bu, C. Vitamin K Status and Cardiovascular Events or Mortality: A Meta-Analysis. Eur. J. Prev. Cardiol. 2019, 26, 549–553. [Google Scholar] [CrossRef]
- Vervoort, L.M.; Ronden, J.E.; Thijssen, H.H. The Potent Antioxidant Activity of the Vitamin K Cycle in Microsomal Lipid Peroxidation. Biochem. Pharmacol. 1997, 54, 871–876. [Google Scholar] [CrossRef]
- Dihingia, A.; Ozah, D.; Ghosh, S.; Sarkar, A.; Baruah, P.K.; Kalita, J.; Sil, P.C.; Manna, P. Vitamin K1 Inversely Correlates with Glycemia and Insulin Resistance in Patients with Type 2 Diabetes (T2D) and Positively Regulates SIRT1/AMPK Pathway of Glucose Metabolism in Liver of T2D Mice and Hepatocytes Cultured in High Glucose. J. Nutr. Biochem. 2018, 52, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Asadipooya, K.; Graves, L.; Lukert, B.P.; Kalantarhormozi, M.; Assadi, M.; Ostovar, A.; Larijani, B.; Nabipour, I. Osteocalcin Is a Predictor for Diabetes Mellitus in Postmenopausal Women and Correlated with Oral Intake of Vitamin K. Mediterr. J. Nutr. Metab. 2015, 8, 231–241. [Google Scholar] [CrossRef]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y.; et al. Endocrine Regulation of Energy Metabolism by the Skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef]
- Ibarrola-Jurado, N.; Salas-Salvadó, J.; Martínez-González, M.A.; Bulló, M. Dietary Phylloquinone Intake and Risk of Type 2 Diabetes in Elderly Subjects at High Risk of Cardiovascular Disease. Am. J. Clin. Nutr. 2012, 96, 1113–1118. [Google Scholar] [CrossRef]
- Rasekhi, H.; Karandish, M.; Jalali, M.T.; Mohammadshahi, M.; Zarei, M.; Saki, A.; Shahbazian, H. Phylloquinone Supplementation Improves Glycemic Status Independent of the Effects of Adiponectin Levels in Premonopause Women with Prediabetes: A Double-Blind Randomized Controlled Clinical Trial. J. Diabetes Metab. Disord. 2015, 14, 1. [Google Scholar] [CrossRef]
- Rasekhi, H.; Karandish, M.; Jalali, M.T.; Mohammad-Shahi, M.; Zarei, M.; Saki, A.; Shahbazian, H. The Effect of Vitamin K1 Supplementation on Sensitivity and Insulin Resistance via Osteocalcin in Prediabetic Women: A Double-Blind Randomized Controlled Clinical Trial. Eur. J. Clin. Nutr. 2015, 69, 891–895. [Google Scholar] [CrossRef]
- Assimacopoulos-Jeannet, F. Fat Storage in Pancreas and in Insulin-Sensitive Tissues in Pathogenesis of Type 2 Diabetes. Int. J. Obes. Relat. Metab. Disord. 2004, 28 (Suppl. S4), S53–S57. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Binkley, N.; Vella, A. Effect of Phylloquinone Supplementation on Glucose Homeostasis in Humans. Am. J. Clin. Nutr. 2010, 92, 1528–1532. [Google Scholar] [CrossRef] [PubMed]

| Reference | Study Type | Subjects | Conclusion (Key Finding) |
|---|---|---|---|
| Vitamin A | |||
| [10] | Cohort study | Postmenopausal women, aged 50–79 y, n = 153 | Lower risks of diabetes were associated with a higher intake of α- and β-carotene. |
| [11] | Prospective study | Human, n = 37,846 | Carotenoids are known to have antioxidant functions, which may underlie the observed inverse associations with diabetes. |
| [12] | Randomized controlled trial | Male smokers aged 50–69 y, n = 29,133 | Supplementation with α-tocopherol or β-carotene had no preventive effect on the risk of type 2 diabetes in middle-aged male smokers. |
| [13] | Prospective study | Adults without cardiovascular disease, aged 45–84 y, n = 6814 | β-carotene was not associated with the risk of T2D. |
| [14] | Prospective cohort study | Healthy Japanese, aged 40–79 y, n = 19,168 | A higher dietary intake of fat-soluble vitamins K and E, but not vitamin A or D, was associated with lowered risk of type 2 diabetes among the Japanese population. |
| [15] | Prospective cohort study | Adults (8537 men and 8577 women), n = 17,111 | The adequate intake of vitamin A may help protect against diabetes, especially in men. |
| [16] | Animal experiment | Dietary VA deficiency model mice | VA deficiency induced pancreatic islets dysfunction by activating the ISC population. |
| [17] | Animal experiment | C57BL/6, LRAT−/− (lecithin retinol acyltransferase null) | VA is essential for the maintenance of β-cell functions in adult pancreas. |
| [18] | Prospective study | Prediabetes, n = 1011 | A U-shaped relationship existed between serum retinol-binding protein 4 levels (>55 μg/mL or <31 μg/mL) and the risk of incident type 2 diabetes in subjects with prediabetes. |
| [19] | Animal experiment | Transgenic mice expressing human RBP4 | Retinol-binding protein 4 represses insulin synthesis through the STRA6/JAK2/STAT1/ISL-1 signaling pathway. |
| Vitamin B | |||
| [20] | Randomized controlled trial | Patients with diabetes, n = 31 | The combined administration of vitamins B1 and B6 to diabetic nephropathy patients causes a decrease in DNA glycation in leukocytes. |
| [21] | Cross-sectional study | Microalbuminurics type 2 diabetics (n = 20), healthy individuals, n = 20, macroalbuminuric type 2 diabetics, n = 20 | Thiamine levels were reduced in the diabetic population, and this reduction in thiamine levels was negatively correlated with the lipid profile in microalbuminuric diabetics. |
| [22] | Animal experiment | Vitamin B6-deficient rats | Vitamin B6 supplementation could normalize urinary 3-hydroxykynurenine and xanthurenic levels in rats. |
| [23] | Cross-sectional studies, animal experiment, cell experiments | Participants (BMI 20–68 kg/m2); 3T3-L1, SAT and VAT adipocyte cells | These results support the notion that the in situ production of PLP is required for physiological adipogenesis. |
| [24] | Cell experiments | 3T3-L1 adipocyte cells | Vitamin B6 can act as an activator for PPARγ, which may contribute to the antitumor and anti-inflammatory effects of vitamin B6. |
| [25] | Animal experiment | Mice with high-fat diet (HFD) | Vitamin B6 protects endothelial function and improves insulin resistance, and low Vitamin B6 status might be a risk factor for NAFLD. |
| Vitamins C and E | |||
| [26] | Meta-analysis | Type 2 diabetic patients and vitamin C as key words | There is no adequate evidence to support vitamin C supplementation for dyslipidemias in diabetic patients. Specific groups of patients might have benefited, including younger diabetic patients. |
| [27] | Meta-analysis | Randomized controlled trials related to diabetes | Vitamin C supplementation may be potentially effective for improving glycemic control and BP in people with type 2 diabetes. |
| [28] | Meta-analysis | Randomized controlled trials related to diabetes | The administration of chromium, CoQ10, vitamin C and vitamin E as add-on supplements for patients with T2DM resulted in significant effects on important glycemic control parameters. |
| [29] | Randomized controlled trial | T2DM male patients, n = 40 | Antioxidant vitamin supplementation (VC/VE) can improve the clinical status of type 2 diabetes mellitus and reduce or prevent the pathogenesis and complications of diabetes. |
| [30] | Randomized controlled trial | Type 2 diabetic patients, n = 50 | Vitamin C may have beneficial effects on HDL-C in diabetic patients, without having significant effects on plasma glucose or other lipid parameters. |
| [31] | Randomized controlled trial | Patients with T2DM mellitus, n = 70 | Vitamin C is a powerful adjunct to the treatment of T2DM. |
| [32] | Randomized controlled trial | T2DM patients, n = 40 | Chronic vitamin C administration has beneficial effects upon glucose and lipid metabolism in aged non-insulin-dependent (type II) diabetic patients. |
| [33] | Animal experiment | Diabetic rats | Vitamin E can regulate the activity of phospholipase A(2) and PLA(2), reduce the production of reactive oxygen species and destructive oxides and maintain the fluidity of liver cell membrane in diabetic rats. |
| [34] | Animal experiment | Male Wistar rats | Antioxidants (VC) reduce insulin resistance induced by obesity/dyslipidemia in humans. |
| [35] | Animal experiment | OB/OB mice | Vitamin C supplementation can decrease insulin glycation and ameliorate aspects of the obesity–diabetes syndrome in ob/ob mice. |
| [36] | Randomized controlled trial | Participants with least three features of metabolic syndrome, n = 80 | Combined supplementation of α-tocopherol AT + γ-tocopherol GT was able to reduce oxidative and nitration stress and inflammation levels in subjects with metabolic syndrome. |
| [37] | Meta-analysis | Vitamin C + glucose + insulin + HbA1c | Age, baseline BMI, plasma glucose levels and effect size were the modifiers of the effect of vitamin C on insulin concentration. |
| Vitamin D | |||
| [38] | Retrospective study | Children with T1DM, n = 1148 | The study supports the concept of seasonality in T1DM diagnosis, implying a possible relationship between clinical expression of T1DM and various climatic factors. |
| [39] | Retrospective study | Children with diabetes, n = 2166 | There is a significant seasonal variation in the incidence of T1DM in Danish children, which may be related to virus prevalence, sun exposure or vitamin D levels. |
| [40] | Prospective study | Pregnant women, n = 2658 | The polymorphisms of the VD metabolic pathway gene were associated with gestational 25(OH)D, and the associations differ by seasons and VD supplements. |
| [41] | Cell experiment; Animal experiment | Db/db mice; β cell | Activation of VDR combined with the dismissal of the BAF complex is able to improve β-cell function and thereby glucose homeostasis in an inflammation-driven diabetes model. |
| [42] | Animal experiment | Insulin resistance rat model | This is the first study highlighting the unprecedented role of 1,25-D3 (short-term effect) in the regulation of glucose homeostasis and on the prevention of insulin resistance. |
| [43] | Randomized controlled trial | Children with T1D, n = 42 | Sustained serum 25-(OH)D concentrations with cholecalciferol supplementation for one year improves metabolic control and slows the decline of RBCF in children with T1DM. |
| [44] | Randomized controlled trial | Children with T1D, n = 52 | Oral vitamin D may serve as an adjuvant to insulin therapy for children with T1DM by augmenting residual beta-cell function and improving insulin secretion. |
| [45] | Randomized controlled trial | Adults with prediabetes, n = 2423 | Among persons at high risk for T2DM not selected for vitamin D insufficiency, vitamin D3 supplementation at a dose of 4000 IU per day did not result in a significantly lower risk of diabetes than the placebo. |
| [46] | Animal experiment | Vitamin D deficiency rats | Pancreases from vitamin D-deficient rats exhibited a 48 percent reduction in insulin secretion compared to that for pancreases from vitamin D-deficient rats that had been replenished with vitamin D. |
| [47] | Randomized controlled trial | Participants with T2DM, n = 96 | Vitamin D supplementation significantly improves peripheral insulin sensitivity and β-cell function, suggesting that it may slow metabolic deterioration in this population. |
| Vitamin K | |||
| [48] | Cross-sectional analysis | Men (aged 55–80), women (aged 60–80 years), n = 568 | Increasing dietary phylloquinone intake reduces inflammation and inflammation-related molecules. |
| [49] | Cross-sectional analysis | Human, aged 26–81, n = 2719 | Phylloquinone has beneficial effects on glucose homeostasis in both men and women. |
| [50] | Prospective cohort study | Human, aged 20–70 y, n = 38,094 | Phylloquinone and menaquinones intakes may be associated with a reduced risk of T2DM. |
| [51] | Randomized controlled trial | Man, aged 21, n = 12 | Appropriate supplementation of vitamin K can regulate insulin metabolism, and the increase in vitamin K intake is associated with the decrease in immunoreactive insulin. |
| [52] | Randomized controlled trial | Healthy man, n = 42 | Vitamin K2 may have a role in enhancing insulin sensitivity in healthy men. |
| [53] | Mendelian randomization | Patients with T2DM, n = 69,647, participants without diabetes, n = 51,336 | The circulating VK1 concentration may be associated with the reduced risk of T2DM at the genetic level. |
| [54] | Animal experiment | C57BL/6J, fill with osteocalcin | Osteocalcin could promote β-cell proliferation and insulin secretion, as well as increase insulin sensitivity. |
| [55] | Cell experiment | Human monocytic THP-1 and mouse RAW264.7 cells | The anti-inflammatory activity of vitamin K is mediated via the inactivation of the NFκB signaling pathway. |
| [56] | Animal experiment | Sprague Dawley rats | Long-term intake of vitamin K2 in SD rats significantly reduced total fat mass and serum glyceride levels, thereby alleviating insulin resistance. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Qin, L.; Zheng, J.; Tong, L.; Lu, W.; Lu, C.; Sun, J.; Fan, B.; Wang, F. Research Progress on the Relationship between Vitamins and Diabetes: Systematic Review. Int. J. Mol. Sci. 2023, 24, 16371. https://doi.org/10.3390/ijms242216371
Liu J, Qin L, Zheng J, Tong L, Lu W, Lu C, Sun J, Fan B, Wang F. Research Progress on the Relationship between Vitamins and Diabetes: Systematic Review. International Journal of Molecular Sciences. 2023; 24(22):16371. https://doi.org/10.3390/ijms242216371
Chicago/Turabian StyleLiu, Jiameng, Luqi Qin, Jiahuan Zheng, Litao Tong, Wei Lu, Cong Lu, Jing Sun, Bei Fan, and Fengzhong Wang. 2023. "Research Progress on the Relationship between Vitamins and Diabetes: Systematic Review" International Journal of Molecular Sciences 24, no. 22: 16371. https://doi.org/10.3390/ijms242216371
APA StyleLiu, J., Qin, L., Zheng, J., Tong, L., Lu, W., Lu, C., Sun, J., Fan, B., & Wang, F. (2023). Research Progress on the Relationship between Vitamins and Diabetes: Systematic Review. International Journal of Molecular Sciences, 24(22), 16371. https://doi.org/10.3390/ijms242216371