Impact of Treatment with Antioxidants as an Adjuvant to Standard Therapy in Patients with Septic Shock: Analysis of the Correlation between Cytokine Storm and Oxidative Stress and Therapeutic Effects
Abstract
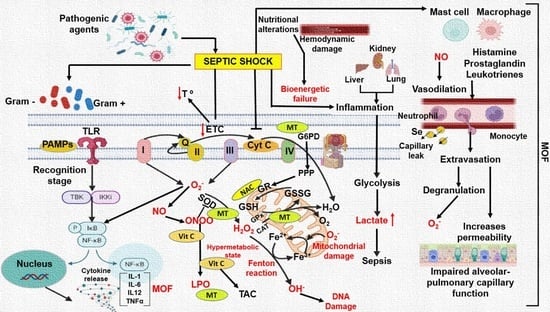
:1. Introduction
2. Results
2.1. Population Studied
2.2. SOFA Score Assessment
2.3. Lipoperoxidation Levels: Enzymatic and Non-Enzymatic Antioxidant Pathways
2.4. Cytokine Quantification
2.5. Canonical Correlation
2.6. Protein–Protein Interaction Network
3. Discussion
4. Methods and Materials
4.1. Study Population
4.2. Sample Size
4.3. Randomization
4.4. Masking and Drug Administration
4.5. Data Collection Method
4.6. Standard Therapy in the ICU
4.7. Sample Collection and Storage
4.8. Oxidative Stress Markers in Plasma
4.8.1. NO3−/NO2− Ratio
4.8.2. GSH Concentration
4.8.3. Evaluation Total Antioxidant Capacity (TAC)
4.8.4. Lipid Peroxidation (LPO)
4.8.5. Carbonylation Protein Concentration
4.8.6. Determination of Selenium (Se)
4.8.7. Thiols
4.8.8. GPx Activity
4.8.9. GST Activity
4.8.10. Extracellular Superoxide Dismutase (ecSOD)
4.8.11. Vitamin C Levels
4.9. Cytokine Measurement
4.10. Statistical Analysis
4.11. Ethical Aspects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M.; for the Sepsis Definitions Task Force. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock. JAMA 2016, 315, 775. [Google Scholar] [CrossRef]
- Marshall, J.C.; Vincent, J.-L.; Guyatt, G.; Angus, D.C.; Abraham, E.; Bernard, G.; Bombardier, C.; Calandra, T.; Jørgensen, H.S.; Sylvester, R.; et al. Outcome measures for clinical research in sepsis: A report of the 2nd Cambridge Colloquium of the International Sepsis Forum. Crit. Care Med. 2005, 33, 1708–1716. [Google Scholar] [CrossRef]
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis. JAMA 2016, 315, 762. [Google Scholar] [CrossRef]
- Alunno, A.; Carubbi, F.; Rodríguez-Carrio, J. Storm, typhoon, cyclone or hurricane in patients with COVID-19? Beware of the same storm that has a different origin. RMD Open 2020, 6, e001295. [Google Scholar] [CrossRef]
- Levin, M. Childhood Multisystem Inflammatory Syndrome—A New Challenge in the Pandemic. N. Engl. J. Med. 2020, 383, 393–395. [Google Scholar] [CrossRef]
- Castagnoli, R.; Votto, M.; Licari, A.; Brambilla, I.; Bruno, R.; Perlini, S.; Rovida, F.; Baldanti, F.; Marseglia, G.L. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Children and Adolescents. JAMA Pediatr. 2020, 174, 882. [Google Scholar] [CrossRef]
- Crimi, E.; Sica, V.; Williams-Ignarro, S.; Zhang, H.; Slutsky, A.S.; Ignarro, L.J.; Napoli, C. The role of oxidative stress in adult critical care. Free Radic. Biol. Med. 2006, 40, 398–406. [Google Scholar] [CrossRef]
- Volk, T.; Kox, W.J. Endothelium function in sepsis. Inflamm. Res. 2000, 49, 185–198. [Google Scholar] [CrossRef]
- Letessier, W.; Demaret, J.; Gossez, M.; Allam, C.; Venet, F.; Rimmelé, T.; Monneret, G. Decreased intra-lymphocyte cytokines measurement in septic shock patients: A proof of concept study in whole blood. Cytokine 2018, 104, 78–84. [Google Scholar] [CrossRef]
- Roth, G.; Moser, B.; Krenn, C.; Brunner, M.; Haisjackl, M.; Almer, G.; Gerlitz, S.; Wolner, E.; Boltz-Nitulescu, G.; Ankersmit, H.J. Susceptibility to programmed cell death in T-lymphocytes from septic patients: A mechanism for lymphopenia and Th2 predominance. Biochem. Biophys. Res. Commun. 2003, 308, 840–846. [Google Scholar] [CrossRef]
- Cohen, J. The immunopathogenesis of sepsis. Nature 2002, 420, 885–891. [Google Scholar] [CrossRef]
- Chao, J.; Cui, S.; Liu, C.; Liu, S.; Liu, S.; Han, Y.; Gao, Y.; Ge, D.; Yu, A.; Yang, R. Detection of early cytokine storm in patients with septic shock after abdominal surgery. J. Transl. Int. Med. 2020, 8, 91–98. [Google Scholar] [CrossRef]
- Liu, X.; Zhan, Z.; Li, D.; Xu, L.; Ma, F.; Zhang, P.; Yao, H.; Cao, X. Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining activation of the kinase Btk. Nat. Immunol. 2011, 12, 416–424. [Google Scholar] [CrossRef]
- Liang, Y.; Li, X.; Zhang, X.; Li, Z.; Wang, L.; Sun, Y.; Liu, Z.; Ma, X. Elevated Levels of Plasma TNF-α Are Associated with Microvascular Endothelial Dysfunction in Patients with Sepsis Through Activating the NF-κB and p38 Mitogen-Activated Protein Kinase in Endothelial Cells. Shock 2014, 41, 275–281. [Google Scholar] [CrossRef]
- Hack, C.E.; De Groot, E.R.; Felt-Bersma, R.J.; Nuijens, J.H.; Strack Van Schijndel, R.J.; Eerenberg-Belmer, A.J.; Thijs, L.G.; Aarden, L.A. Increased plasma levels of interleukin-6 in sepsis. Blood 1989, 74, 1704–1710. [Google Scholar] [CrossRef]
- Miguel-Bayarri, V.; Casanoves-Laparra, E.B.; Pallás-Beneyto, L.; Sancho-Chinesta, S.; Martín-Osorio, L.F.; Tormo-Calandín, C.; Bautista-Rentero, D. Valor pronóstico de los biomarcadores procalcitonina, interleukina 6 y proteína C reactiva en la sepsis grave. Med. Intensiv. 2012, 36, 556–562. [Google Scholar] [CrossRef]
- Xie, Y.; Li, B.; Lin, Y.; Shi, F.; Chen, W.; Wu, W.; Zhang, W.; Fei, Y.; Zou, S.; Yao, C. Combining Blood-Based Biomarkers to Predict Mortality of Sepsis at Arrival at the Emergency Department. Med. Sci. Monit. 2020, 27, e929527-1. [Google Scholar] [CrossRef]
- Hack, C.E.; Zeerleder, S. The endothelium in sepsis: Source of and a target for inflammation. Crit. Care Med. 2001, 29, S21–S27. [Google Scholar] [CrossRef]
- Marshall, J.C. Inflammation, coagulopathy, and the pathogenesis of multiple organ dysfunction syndrome. Crit. Care Med. 2001, 29, S99–S106. [Google Scholar] [CrossRef]
- Lehrnbecher, T.; Venzon, D.; de Haas, M.; Chanock, S.J.; Kühl, J. Assessment of Measuring Circulating Levels of Interleukin-6, Interleukin-8, C-Reactive Protein, Soluble Fcy Receptor Type III, and Mannose-Binding Protein in Febrile Children with Cancer and Neutropenia. Clin. Infect. Dis. 1999, 29, 414–419. [Google Scholar] [CrossRef]
- Spasova, M.I.; Terzieva, D.D.; Tzvetkova, T.Z.; Stoyanova, A.A.; Mumdzhiev, I.N.; Yanev, I.B.; Genev, E.D. Interleukin-6, interleukin-8, interleukin-10, and C-reactive protein in febrile neutropenia in children with malignant diseases. Folia Med. 2005, 47, 46–52. [Google Scholar]
- Borrelli, E.; Roux-Lombard, P.; Grau, G.E.; Girardin, E.; Ricou, B.; Dayer, J.-M.; Suter, P.M. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit. Care Med. 1996, 24, 392–397. [Google Scholar] [CrossRef]
- Maier, B.; Lefering, R.; Lehnert, M.; Laurer, H.L.; Steudel, W.I.; Neugebauer, E.A.; Marzi, I. Early versus late onset of multiple organ failure is associated with differing patterns of plasma cytokine biomarker expression and outcome after severe trauma. Shock 2007, 28, 668–674. [Google Scholar] [CrossRef]
- Damas, P.; Canivet, J.L.; de Groote, D.; Vrindts, Y.; Albert, A.; Franchimont, P.; Lamy, M.M. Sepsis and serum cytokine concentrations. Crit. Care Med. 1997, 25, 405–412. [Google Scholar] [CrossRef]
- Razazi, K.; Boissier, F.; Surenaud, M.; Bedet, A.; Seemann, A.; Carteaux, G.; de Prost, N.; Brun-Buisson, C.; Hue, S.; Dessap, A.M. A multiplex analysis of sepsis mediators during human septic shock: A preliminary study on myocardial depression and organ failures. Ann. Intensive Care 2019, 9, 64. [Google Scholar] [CrossRef]
- Reinhart, K.; Bayer, O.; Brunkhorst, F.; Meisner, M. Markers of endothelial damage in organ dysfunction and sepsis. Crit. Care Med. 2002, 30, S302–S312. [Google Scholar] [CrossRef]
- Nedel, W.L.; Strogulski, N.R.; Rodolphi, M.S.; Kopczynski, A.; Montes, T.H.M.; Portela, L.V. Short-term inflammatory biomarker profiles are associated with deficient mitochondrial bioenergetics in lymphocytes of septic shock patients—A prospective cohort study. Shock 2023, 59, 288–293. [Google Scholar] [CrossRef]
- Donoso, A.; Arriagada-Santis, D. Síndrome de disfunción de órganos y adaptación mitocondrial en el paciente séptico. Bol. Med. Hosp. Infant. Mex. 2021, 78, 597–611. [Google Scholar] [CrossRef]
- Taniguchi, T.; Koido, Y.; Aiboshi, J.; Yamashita, T.; Suzaki, S.; Kurokawa, A. Change in the ratio of interleukin-6 to interleukin-10 predicts a poor outcome in patients with systemic inflammatory response syndrome. Crit. Care Med. 1999, 27, 1262–1264. [Google Scholar] [CrossRef]
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef]
- Ayala, A.; Wesche-Soldato, D.E.; Perl, M.; Lomas-Neira, J.L.; Swan, R.; Chung, C.-S. Blockade of apoptosis as a rational therapeutic strategy for the treatment of sepsis. Novartis Found. Symp. 2007, 280, 37–49; discussion 49–52, 160–164. [Google Scholar] [CrossRef]
- Rittirsch, D.; Flierl, M.A.; Ward, P.A. Harmful molecular mechanisms in sepsis. Nat. Rev. Immunol. 2008, 8, 776–787. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Reczek, C.R.; Chandel, N.S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 2015, 33, 8–13. [Google Scholar] [CrossRef]
- Li, F.; Yan, R.; Wu, J.; Han, Z.; Qin, M.; Liu, C.; Lu, Y. An Antioxidant Enzyme Therapeutic for Sepsis. Front. Bioeng. Biotechnol. 2021, 9, 800684. [Google Scholar] [CrossRef]
- Wesche-Soldato, D.E.; Swan, R.Z.; Chung, C.-S.; Ayala, A. The apoptotic pathway as a therapeutic target in sepsis. Curr. Drug Targets 2007, 8, 493–500. [Google Scholar] [CrossRef]
- Manchikalapati, R.; Schening, J.; Farias, A.J.; Sacco, K. Clinical Utility of Interleukin-1 Inhibitors in Pediatric Sepsis. Shock 2023. [Google Scholar] [CrossRef]
- Hu, B.; Chen, Z.; Liang, L.; Zheng, M.; Chen, X.; Zeng, Q. Melatonin Promotes Mitochondrial Biogenesis and Mitochondrial Degradation in Hepatocytes During Sepsis. Altern. Ther. Health Med. 2023, 29, 284–289. [Google Scholar]
- Hadi, S.M.H.; Majeed, S.; Ghafil, F.A.; Altoraihi, K.; Hadi, N.R. Effect of Sulforaphane on cardiac injury induced by sepsis in a mouse model: Role of toll-like receptor 4. J. Med. Life 2023, 16, 1120–1126. [Google Scholar] [CrossRef]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the Eye of the Cytokine Storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef]
- Dare, A.J.; Phillips, A.R.J.; Hickey, A.J.R.; Mittal, A.; Loveday, B.; Thompson, N.; Windsor, J.A. A systematic review of experimental treatments for mitochondrial dysfunction in sepsis and multiple organ dysfunction syndrome. Free Radic. Biol. Med. 2009, 47, 1517–1525. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar] [CrossRef]
- Joseph, L.C.; Kokkinaki, D.; Valenti, M.-C.; Kim, G.J.; Barca, E.; Tomar, D.; Hoffman, N.E.; Subramanyam, P.; Colecraft, H.M.; Hirano, M.; et al. Inhibition of NADPH oxidase 2 (NOX2) prevents sepsis-induced cardiomyopathy by improving calcium handling and mitochondrial function. JCI Insight 2017, 2, e94248. [Google Scholar] [CrossRef]
- Virdis, A.; Colucci, R.; Fornai, M.; Blandizzi, C.; Duranti, E.; Pinto, S.; Bernardini, N.; Segnani, C.; Antonioli, L.; Taddei, S.; et al. Cyclooxygenase-2 Inhibition Improves Vascular Endothelial Dysfunction in a Rat Model of Endotoxic Shock: Role of Inducible Nitric-Oxide Synthase and Oxidative Stress. J. Pharmacol. Exp. Ther. 2005, 312, 945–953. [Google Scholar] [CrossRef]
- Jacobi, J.; Kristal, B.; Chezar, J.; Shaul, S.M.; Sela, S. Exogenous superoxide mediates pro-oxidative, proinflammatory, and procoagulatory changes in primary endothelial cell cultures. Free Radic. Biol. Med. 2005, 39, 1238–1248. [Google Scholar] [CrossRef]
- Rondovic, G.; Djordjevic, D.; Udovicic, I.; Stanojevic, I.; Zeba, S.; Abazovic, T.; Vojvodic, D.; Abazovic, D.; Khan, W.; Surbatovic, M. From Cytokine Storm to Cytokine Breeze: Did Lessons Learned from Immunopathogenesis Improve Immunomodulatory Treatment of Moderate-to-Severe COVID-19? Biomedicines 2022, 10, 2620. [Google Scholar] [CrossRef]
- Aisa-Álvarez, A.; Pérez-Torres, I.; Guarner-Lans, V.; Manzano-Pech, L.; Cruz-Soto, R.; Márquez-Velasco, R.; Casarez-Alvarado, S.; Franco-Granillo, J.; Núñez-Martínez, M.E.; Soto, M.E. Randomized Clinical Trial of Antioxidant Therapy Patients with Septic Shock and Organ Dysfunction in the ICU: SOFA Score Reduction by Improvement of the Enzymatic and Non-Enzymatic Antioxidant System. Cells 2023, 12, 1330. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef]
- Chen, G.Y.; Nuñez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef]
- Ruiz-Sanmartín, A.; Ribas, V.; Suñol, D.; Chiscano-Camón, L.; Palmada, C.; Bajaña, I.; Larrosa, N.; González, J.J.; Canela, N.; Ferrer, R.; et al. Characterization of a proteomic profile associated with organ dysfunction and mortality of sepsis and septic shock. PLoS ONE 2022, 17, e0278708. [Google Scholar] [CrossRef]
- Bauzá-Martinez, J.; Aletti, F.; Pinto, B.B.; Ribas, V.; Odena, M.A.; Díaz, R.; Romay, E.; Ferrer, R.; Kistler, E.; Tedeschi, G.; et al. Proteolysis in septic shock patients: Plasma peptidomic patterns are associated with mortality. Br. J. Anaesth. 2018, 121, 1065–1074. [Google Scholar] [CrossRef]
- Reinhart, K.; Daniels, R.; Kissoon, N.; Machado, F.R.; Schachter, R.D.; Finfer, S. Recognizing Sepsis as a Global Health Priority—A WHO Resolution. N. Engl. J. Med. 2017, 377, 414–417. [Google Scholar] [CrossRef]
- Cohen, J.; Vincent, J.-L.; Adhikari, N.K.J.; Machado, F.R.; Angus, D.C.; Calandra, T.; Jaton, K.; Giulieri, S.; Delaloye, J.; Opal, S.; et al. Sepsis: A roadmap for future research. Lancet Infect. Dis. 2015, 15, 581–614. [Google Scholar] [CrossRef]
- Cavaillon, J.-M. Exotoxins and endotoxins: Inducers of inflammatory cytokines. Toxicon 2018, 149, 45–53. [Google Scholar] [CrossRef]
- Striz, I.; Brabcova, E.; Kolesar, L.; Sekerkova, A. Cytokine networking of innate immunity cells: A potential target of therapy. Clin. Sci. 2014, 126, 593–612. [Google Scholar] [CrossRef]
- Anderson, G.; Reiter, R.J. Melatonin: Roles in influenza, COVID-19, and other viral infections. Rev. Med. Virol. 2020, 30, e2109. [Google Scholar] [CrossRef]
- Paterson, R.L.; Galley, H.F.; Webster, N.R. The effect of N-acetylcysteine on nuclear factor-κB activation, interleukin-6, interleukin-8, and intercellular adhesion molecule-1 expression in patients with sepsis*. Crit. Care Med. 2003, 31, 2574–2578. [Google Scholar] [CrossRef]
- Soto, M.E.; Guarner-Lans, V.; Soria-Castro, E.; Manzano Pech, L.; Pérez-Torres, I. Is Antioxidant Therapy a Useful Complementary Measure for COVID-19 Treatment? An Algorithm for Its Application. Medicina 2020, 56, 386. [Google Scholar] [CrossRef]
- Rank, N.; Michel, C.; Haertel, C.; Med, C.; Lenhart, A.; Welte, M.; Meier-Hellmann, A.; Spies, C. N-acetylcysteine increases liver blood flow and improves liver function in septic shock patients: Results of a prospective, randomized, double-blind study. Crit. Care Med. 2000, 28, 3799–3807. [Google Scholar] [CrossRef]
- Zaniew, M.; Zachwieja, J.; Lewandowska-Stachowiak, M.; Sobczyk, D.; Siwińska, A. The antioxidant therapy modulates intracellular lymphokine expression in children on dialysis. Przegl. Lek. 2006, 63 (Suppl. S3), 63–67. [Google Scholar]
- Abdelrazik, E.; Hassan, H.M.; Abdallah, Z.; Magdy, A.; Farrag, E.A. Renoprotective effect of N-acetylcystein and vitamin E in bisphenol A-induced rat nephrotoxicity; Modulators of Nrf2/NF-κB and ROS signaling pathway. Acta Biomed. 2022, 93, e2022301. [Google Scholar] [CrossRef]
- Sawoo, R.; Dey, R.; Ghosh, R.; Bishayi, B. Exogenous IL-10 posttreatment along with TLR4 and TNFR1 blockade improves tissue antioxidant status by modulating sepsis-induced macrophage polarization. J. Appl. Toxicol. 2023, 43, 1549–1572. [Google Scholar] [CrossRef]
- Martin, J.G.; Kurokawa, C.S.; Carpi, M.F.; Bonatto, R.C.; Moraes, M.A.; Fioretto, J.R. Interleukin-12 in children with sepsis and septic shock. Rev. Bras. Ter. Intensiv. 2012, 24, 130–136. [Google Scholar] [CrossRef]
- Nansen, A.; Randrup Thomsen, A. Viral Infection Causes Rapid Sensitization to Lipopolysaccharide: Central Role of IFN-αβ. J. Immunol. 2001, 166, 982–988. [Google Scholar] [CrossRef]
- Gaignage, M.; Uyttenhove, C.; Jones, L.L.; Bourdeaux, C.; Chéou, P.; Mandour, M.F.; Coutelier, J.; Vignali, D.A.; Van Snick, J. Novel antibodies that selectively block mouse IL-12 enable the re-evaluation of the role of IL-12 in immune protection and pathology. Eur. J. Immunol. 2021, 51, 1482–1493. [Google Scholar] [CrossRef]
- Weidhase, L.; Wellhöfer, D.; Schulze, G.; Kaiser, T.; Drogies, T.; Wurst, U.; Petros, S. Is Interleukin-6 a better predictor of successful antibiotic therapy than procalcitonin and C-reactive protein? A single center study in critically ill adults. BMC Infect. Dis. 2019, 19, 150. [Google Scholar] [CrossRef]
- Visavadiya, N.P.; Soni, B.; Dalwadi, N. Free radical scavenging and antiatherogenic activities of Sesamum indicum seed extracts in chemical and biological model systems. Food Chem. Toxicol. 2009, 47, 2507–2515. [Google Scholar] [CrossRef]
- Hesse, D.G.; Tracey, K.J.; Fong, Y.; Manogue, K.R.; Palladino, M.A.; Cerami, A.; Shires, G.T.; Lowry, S.F. Cytokine appearance in human endotoxemia and primate bacteremia. Surg. Gynecol. Obstet. 1988, 166, 147–153. [Google Scholar]
- Beutler, B.A.; Milsark, I.W.; Cerami, A. Cachectin/tumor necrosis factor: Production, distribution, and metabolic fate in vivo. J. Immunol. 1985, 135, 3972–3977. [Google Scholar] [CrossRef]
- Michie, H.R.; Manogue, K.R.; Spriggs, D.R.; Revhaug, A.; O’Dwyer, S.; Dinarello, C.A.; Cerami, A.; Wolff, S.M.; Wilmore, D.W. Detection of Circulating Tumor Necrosis Factor after Endotoxin Administration. N. Engl. J. Med. 1988, 318, 1481–1486. [Google Scholar] [CrossRef]
- Waage, A.; Brandtzaeg, P.; Halstensen, A.; Kierulf, P.; Espevik, T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J. Exp. Med. 1989, 169, 333–338. [Google Scholar] [CrossRef]
- Kang, Y.-H.; Brummel, S.E.; Lee, C.-H. Differential effects of transforming growth factor-±1 on lipopolysaccharide induction of endothelial adhesion molecules. Shock 1996, 6, 118–125. [Google Scholar] [CrossRef]
- Basodan, N.; Al Mehmadi, A.E.; Al Mehmadi, A.E.; Aldawood, S.M.; Hawsawi, A.; Fatini, F.; Mulla, Z.M.; Nawwab, W.; Alshareef, A.; Almhmadi, A.H.; et al. Septic Shock: Management and Outcomes. Cureus 2022, 14, e32158. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Angus, D.C.; van der Poll, T. Severe Sepsis and Septic Shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef]
- Gabriel, V.; Grigorian, A.; Nahmias, J.; Pejcinovska, M.; Smith, M.; Sun, B.; Won, E.; Bernal, N.; Barrios, C.; Schubl, S.D. Risk Factors for Post-Operative Sepsis and Septic Shock in Patients Undergoing Emergency Surgery. Surg. Infect. 2019, 20, 367–372. [Google Scholar] [CrossRef]
- Medam, S.; Zieleskiewicz, L.; Duclos, G.; Baumstarck, K.; Loundou, A.; Alingrin, J.; Hammad, E.; Vigne, C.; Antonini, F.; Leone, M. Risk factors for death in septic shock. Medicine 2017, 96, e9241. [Google Scholar] [CrossRef]
- Rech, M.A.; Mosier, M.J.; McConkey, K.; Zelisko, S.; Netzer, G.; Kovacs, E.J.; Afshar, M. Outcomes in Burn-Injured Patients Who Develop Sepsis. J. Burn. Care Res. 2019, 40, 269–273. [Google Scholar] [CrossRef]
- Boehm, D.; Menke, H. Sepsis in Burns—Lessons Learnt from Developments in the Management of Septic Shock. Medicina 2021, 58, 26. [Google Scholar] [CrossRef]
- Nappi, F.; Martuscelli, G.; Bellomo, F.; Avtaar Singh, S.S.; Moon, M.R. Infective Endocarditis in High-Income Countries. Metabolites 2022, 12, 682. [Google Scholar] [CrossRef]
- Babeș, E.E.; Lucuța, D.A.; Petcheși, C.D.; Zaha, A.A.; Ilyes, C.; Jurca, A.D.; Vesa, C.M.; Zaha, D.C.; Babeș, V.V. Clinical Features and Outcome of Infective Endocarditis in a University Hospital in Romania. Medicina 2021, 57, 158. [Google Scholar] [CrossRef]
- Peetermans, M.; de Prost, N.; Eckmann, C.; Norrby-Teglund, A.; Skrede, S.; De Waele, J.J. Necrotizing skin and soft-tissue infections in the intensive care unit. Clin. Microbiol. Infect. 2020, 26, 8–17. [Google Scholar] [CrossRef]
- Bruun, T.; Rath, E.; Madsen, M.B.; Oppegaard, O.; Nekludov, M.; Arnell, P.; Karlsson, Y.; Babbar, A.; Bergey, F.; Itzek, A.; et al. Risk Factors and Predictors of Mortality in Streptococcal Necrotizing Soft-tissue Infections: A Multicenter Prospective Study. Clin. Infect. Dis. 2021, 72, 293–300. [Google Scholar] [CrossRef]
- Kovacs, J.; Gurzu, S.; Jung, J.; Szederjesi, J.; Copotoiu, S.-M.; Copotoiu, R.; Azamfirei, L. Clinico-Pathological Particularities of the Shock-Related Pancreatitis. Pathol. Oncol. Res. 2012, 18, 977–981. [Google Scholar] [CrossRef]
- Coureuil, M.; Join-Lambert, O.; Lecuyer, H.; Bourdoulous, S.; Marullo, S.; Nassif, X. Pathogenesis of Meningococcemia. Cold Spring Harb. Perspect. Med. 2013, 3, a012393. [Google Scholar] [CrossRef]
- Mora, A. A Complicated Case of Rheumatoid Arthritis in Septic Shock. Cureus 2022, 14, e25712. [Google Scholar] [CrossRef]
- Jamal, M.; Bangash, H.I.; Habiba, M.; Lei, Y.; Xie, T.; Sun, J.; Wei, Z.; Hong, Z.; Shao, L.; Zhang, Q. Immune dysregulation and system pathology in COVID-19. Virulence 2021, 12, 918–936. [Google Scholar] [CrossRef]
- Neupane, M.; Kadri, S.S. Nangibotide for precision immunomodulation in septic shock and COVID-19. Lancet Respir. Med. 2023, 11, 855–857. [Google Scholar] [CrossRef]
- Dinarello, C.A. Proinflammatory and Anti-inflammatory Cytokines as Mediators in the Pathogenesis of Septic Shock. Chest 1997, 112, 321S–329S. [Google Scholar] [CrossRef]
- Shapiro, L.; Scherger, S.; Franco-Paredes, C.; Gharamti, A.; Henao-Martinez, A.F. Anakinra authorized to treat severe coronavirus disease 2019; Sepsis breakthrough or time to reflect? Front. Microbiol. 2023, 14, 1250483. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Ni, L.; Di, X.; Ma, B.; Niu, S.; Liu, C.; Reiter, R.J. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 2020, 250, 117583. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.J.; Naji, L.; Fernández-Santos, J.M.; Martín-Lacave, I.; Guerrero, J.M.; Calvo, J.R. Beneficial pleiotropic actions of melatonin in an experimental model of septic shock in mice: Regulation of pro-/anti-inflammatory cytokine network, protection against oxidative damage and anti-apoptotic effects. J. Pineal Res. 2005, 39, 400–408. [Google Scholar] [CrossRef]
- Wang, H. Protective effect of melatonin against liver injury in mice induced by Bacillus Calmette-Guerin plus lipopolysaccharide. World J. Gastroenterol. 2004, 10, 2690. [Google Scholar] [CrossRef]
- Lassnigg, A.; Punz, A.; Barker, R.; Keznickl, P.; Manhart, N.; Roth, E.; Hiesmayr, M. Influence of intravenous vitamin E supplementation in cardiac surgery on oxidative stress: A double-blinded, randomized, controlled study. Br. J. Anaesth. 2003, 90, 148–154. [Google Scholar] [CrossRef]
- Mayo, J.C.; Sainz, R.M.; González Menéndez, P.; Cepas, V.; Tan, D.-X.; Reiter, R.J. Melatonin and sirtuins: A “not-so unexpected” relationship. J. Pineal Res. 2017, 62, e12391. [Google Scholar] [CrossRef]
- Stefulj, J.; Hörtner, M.; Ghosh, M.; Schauenstein, K.; Rinner, I.; Wölfler, A.; Semmler, J.; Liebmann, P.M. Gene expression of the key enzymes of melatonin synthesis in extrapineal tissues of the rat. J. Pineal Res. 2001, 30, 243–247. [Google Scholar] [CrossRef]
- Masana, M.I.; Dubocovich, M.L. Melatonin Receptor Signaling: Finding the Path Through the Dark. Sci. STKE 2001, 2001, pe39. [Google Scholar] [CrossRef]
- Dubocovich, M.L. Molecular pharmacology regulation and function of mammalian melatonin receptors. Front. Biosci. 2003, 8, 1089. [Google Scholar] [CrossRef]
- Tarocco, A.; Caroccia, N.; Morciano, G.; Wieckowski, M.R.; Ancora, G.; Garani, G.; Pinton, P. Melatonin as a master regulator of cell death and inflammation: Molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 2019, 10, 317. [Google Scholar] [CrossRef]
- Aisa-Alvarez, A.; Soto, M.E.; Guarner-Lans, V.; Camarena-Alejo, G.; Franco-Granillo, J.; Martínez-Rodríguez, E.A.; Ávila, R.G.; Pech, L.M.; Pérez-Torres, I. Usefulness of Antioxidants as Adjuvant Therapy for Septic Shock: A Randomized Clinical Trial. Medicina 2020, 56, 619. [Google Scholar] [CrossRef]
- Forceville, X.; Mostert, V.; Pierantoni, A.; Vitoux, D.; Le Toumelin, P.; Plouvier, E.; Dehoux, M.; Thuillier, F.; Combes, A. Selenoprotein P, Rather than Glutathione Peroxidase, as a Potential Marker of Septic Shock and Related Syndromes. Eur. Surg. Res. 2009, 43, 338–347. [Google Scholar] [CrossRef]
- Manthous, C.A.; Hall, J.B.; Samsel, R.W. Endotoxin in Human Disease. Chest 1993, 104, 1872–1881. [Google Scholar] [CrossRef]
- Stephens, R.; Mythen, M. Endotoxin immunization. Intensive Care Med. 2000, 26, S129–S136. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Wicha, P.; Tocharus, J.; Janyou, A.; Jittiwat, J.; Changtam, C.; Suksamrarn, A.; Tocharus, C. Hexahydrocurcumin protects against cerebral ischemia/reperfusion injury, attenuates inflammation, and improves antioxidant defenses in a rat stroke model. PLoS ONE 2017, 12, e0189211. [Google Scholar] [CrossRef]
- Pita Rodríguez, G. Funciones de la Vitamina E en la nutrición humana/Role of vitamin E in human nutrition. Rev. Cuba. Aliment. Nutr. 1997, 11, 46–57. [Google Scholar]
- Tahir, M.; Foley, B.; Pate, G.; Crean, P.; Moore, D.; McCarroll, N.; Walsh, M. Impact of vitamin E and C supplementation on serum adhesion molecules in chronic degenerative aortic stenosis: A randomized controlled trial. Am. Heart J. 2005, 150, 302–306. [Google Scholar] [CrossRef]
- Haileselassie, B.; Joshi, A.U.; Minhas, P.S.; Mukherjee, R.; Andreasson, K.I.; Mochly-Rosen, D. Mitochondrial dysfunction mediated through dynamin-related protein 1 (Drp1) propagates impairment in blood brain barrier in septic encephalopathy. J. Neuroinflamm. 2020, 17, 36. [Google Scholar] [CrossRef]
- Riedel, S.; Carroll, K.C. Laboratory Detection of Sepsis. Clin. Lab. Med. 2013, 33, 413–437. [Google Scholar] [CrossRef]
- Menon, V.; Mohamed, Z.U.; Prasannan, P.; Moni, M.; Edathadathil, F.; Prasanna, P.; Menon, A.; Nair, S.; Greeshma, C.; Sathyapalan, D.T.; et al. Vitamin C Therapy for Routine Care in Septic Shock (ViCTOR) Trial: Effect of Intravenous Vitamin C, Thiamine, and Hydrocortisone Administration on Inpatient Mortality among Patients with Septic Shock. Indian J. Crit. Care Med. 2020, 24, 653–661. [Google Scholar] [CrossRef]
- Fowler, A.A.; Truwit, J.D.; Hite, R.D.; Morris, P.E.; DeWilde, C.; Priday, A.; Fisher, B.; Thacker, L.R., 2nd; Natarajan, R.; Brophy, D.F.; et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure. JAMA 2019, 322, 1261. [Google Scholar] [CrossRef]
- Lowes, D.A.; Almawash, A.M.; Webster, N.R.; Reid, V.L.; Galley, H.F. Melatonin and structurally similar compounds have differing effects on inflammation and mitochondrial function in endothelial cells under conditions mimicking sepsis. Br. J. Anaesth. 2011, 107, 193–201. [Google Scholar] [CrossRef]
- Howe, K.P.; Clochesy, J.M.; Goldstein, L.S.; Owen, H. Mechanical Ventilation Antioxidant Trial. Am. J. Crit. Care 2015, 24, 440–445. [Google Scholar] [CrossRef]
- Khwannimit, B. A comparison of three organ dysfunction scores: MODS, SOFA and LOD for predicting ICU mortality in critically ill patients. J. Med. Assoc. Thail. 2007, 90, 1074–1081. [Google Scholar]
- Soto, M.E.; Manzano-Pech, L.G.; Guarner-Lans, V.; Díaz-Galindo, J.A.; Vásquez, X.; Castrejón-Tellez, V.; Gamboa, R.; Huesca, C.; Fuentevilla-Alvárez, G.; Pérez-Torres, I. Oxidant/Antioxidant Profile in the Thoracic Aneurysm of Patients with the Loeys-Dietz Syndrome. Oxid. Med. Cell Longev. 2020, 2020, 5392454. [Google Scholar] [CrossRef]
- Zúñiga-Muñoz, A.M.; Pérez-Torres, I.; Guarner-Lans, V.; Núñez-Garrido, E.; Velázquez Espejel, R.; Huesca-Gómez, C.; Gamboa-Ávila, R.; Soto, M.E. Glutathione system participation in thoracic aneurysms from patients with Marfan syndrome. Vasa 2017, 46, 177–186. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Erel, O.; Neselioglu, S. A novel and automated assay for thiol/disulphide homeostasis. Clin. Biochem. 2014, 47, 326–332. [Google Scholar] [CrossRef]
- Soto, M.E.; Guarner-Lans, V.; Díaz-Díaz, E.; Manzano-Pech, L.; Palacios-Chavarría, A.; Valdez-Vázquez, R.R.; Aisa-Álvarez, A.; Saucedo-Orozco, H.; Pérez-Torres, I. Hyperglycemia and Loss of Redox Homeostasis in COVID-19 Patients. Cells 2022, 11, 932. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. [12] Assays of Glutathione Peroxidase; Academic Press: Cambridge, MA, USA, 1984; pp. 114–120. [Google Scholar] [CrossRef]
- Soto, M.E.; Soria-Castro, E.; Lans, V.G.; Ontiveros, E.M.; Mejía, B.I.H.; Hernandez, H.J.M.; García, R.B.; Herrera, V.; Pérez-Torres, I. Analysis of Oxidative Stress Enzymes and Structural and Functional Proteins on Human Aortic Tissue from Different Aortopathies. Oxid. Med. Cell Longev. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Jagota, S.K.; Dani, H.M. A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal. Biochem. 1982, 127, 178–182. [Google Scholar] [CrossRef]
- LYSOMUCIL—PLM. Available online: https://www.medicamentosplm.com/Home/productos/lysomucil_tabletas_efervescentes/42/101/36846/218 (accessed on 26 June 2020).
- REDOXON—PLM. Available online: https://www.medicamentosplm.com/Home/productos/redoxon_tabletas_masticables/21/101/9525/220 (accessed on 26 June 2020).
- ETERNAL CÁPSULAS 400 UI de México. Available online: https://www.vademecum.es/mexico/medicamento/1280368/eternal+capsulas+400+ui (accessed on 2 September 2023).
- BENEDORM—PLM. Available online: https://www.medicamentosplm.com/Home/productos/benedorm_tabletas_sublinguales/187/101/10598/224 (accessed on 26 June 2020).
- Johnson, C.E.; Cober, M.P.; Thome, T.; Rouse, E. Stability of an extemporaneous alcohol-free melatonin suspension. Am. J. Health Pharm. 2011, 68, 420–423. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Vitamin E|C29H50O2; National Center for Biotechnology Information: Bethesda, MD, USA, 2022.
- Galli, F.; Azzi, A.; Birringer, M.; Cook-Mills, J.M.; Eggersdorfer, M.; Frank, J.; Cruciani, G.; Lorkowski, S.; Özer, N.K. Vitamin E: Emerging aspects and new directions. Free Radic. Biol. Med. 2017, 102, 16–36. [Google Scholar] [CrossRef]
- Outline, C.; Concepts, A. Distribution in Foods. 2012; pp. 181–211. Available online: https://health2016.globalchange.gov/food-safety-nutrition-and-distribution (accessed on 9 October 2023).





| Vit C n = 25 | Vit E n = 27 | NAC n = 24 | MT. n = 26 | Control n = 29 | p | |
|---|---|---|---|---|---|---|
| Age | 62 (58–78) | 70 (51–77) | 68.5 (58.5–78) | 62.5 (58–69) | 75 (65–81) | 0.10 |
| Weight, kg | 70 (65–80) | 71 (60–82) | 69.5 (56.5–80) | 66 (60–78) | 70 (61–80) | 0.80 |
| Height | 1.7 (1.5–1.7) | 1.7 (1.6–1.7) | 1.7 (1.6–1.8) | 1.7 (1.6–1.71) | 1.7 (1.6–1.7) | 0.39 |
| Body mass index | 24.9 (23–30.1) | 24.6 (22.8–29) | 23 (20.7–6.1) | 25.35 (21–28) | 25 (23.4–28.6) | 0.41 |
| Male/Female | 10 (40)/15 (60) | 17 (62.96)/10 (37.04) | 14 (58.33)/10 (41.67) | 13 (50)/13 (50) | 16 (55.17)/13 (44.83) | 0.53 § |
| SAPSII | 39.44 ± 14.10 | 45.70 ± 16.37 | 42.41 ± 19.84 | 40.88 ± 16.80 | 47.20 ± 7.11 | 0.60 ¥ |
| APACHEII | 14 (12–19) | 20 (15–24) | 15.5 (11–20.5) | 16.5 (10–21) | 17 (15–25) | 0.15 |
| SOFA | 8 (6–9) | 9 (7–11) | 8 (4–10) | 8 (6–9) | 9 (7–11) | 0.42 |
| NUTRIC | 4.16 ± 2.21 | 4.81 ± 1.64 | 4.08 ± 1.83 | 3.88 ± 1.79 | 5.10 ± 1.54 | 0.41 ¥ |
| Diabetes mellitus | 7 (28.00) | 5 (18.52) | 5 (20.83) | 6 (23.08) | 8 (27.59) | 0.90 * |
| Arterial hypertension | 10 (40.00) | 11 (40.74) | 12 (50.00) | 8 (30.77) | 15 (51.72) | 0.53 § |
| COPD | 1 (4.00) | 5 (18.52) | 4 (16.67) | 2 (7.69) | 0 (0.00) | 0.05 * |
| Smoking | 17 (68.00) | 12 (44.44) | 9 (37.50) | 15 (57.69) | 14 (48.28) | 0.22 § |
| Cancer | 6 (24.00) | 11 (40.74) | 8 (33.33) | 8 (30.77) | 14 (48.28) | 0.39 § |
| Cirrhosis | 2 (8.00) | 2 (7.41) | 1 (4.17) | 1 (3.85) | 4 (13.79) | 0.71 * |
| CKD | 2 (8.00) | 3 (11.11) | 4 (16.67) | 3 (11.54) | 3 (10.34) | 0.92 * |
| Hypothyroidism | 4 (16.00) | 4 (14.81) | 2 (8.33) | 6 (23.08) | 7 (24.14) | 0.56 * |
| CVD | 3 (12.00) | 0 (0.00) | 1 (4.17) | 2 (7.69) | 3 (10.34) | 0.41 * |
| Heart stroke | 1 (4.00) | 0 (0.00) | 3 (12.50) | 2 (7.69) | 2 (6.90) | 0.43 * |
| Atrial fibrillation | 3 (12.00) | 2 (7.41) | 3 (12.50) | 5 (19.23) | 4 (13.79) | 0.79 * |
| DVT | 0 (0.00) | 0 (0.00) | 1 (4.17) | 0 (0.00) | 2 (6.90) | 0.39 * |
| PE | 0 (0.00) | 0 (0.00) | 2 (8.33) | 1 (3.85) | 2 (6.90) | 0.42 * |
| Characteristics | Vit C n = 25 | Vit E n = 27 | NAC n = 24 | MT. n = 26 | Control n = 29 | p |
|---|---|---|---|---|---|---|
| Reason for admission, n (%) | ||||||
| Surgical | 7 (28.00) | 6 (22.22) | 5 (20.83) | 4 (15.38) | 13 (44.83) | 0.14 |
| Non-surgical | 18 (72.00) | 21 (77.78) | 19 (79.17) | 22 (84.62) | 16 (55.17) | |
| Place of admission, n (%) | ||||||
| Emergency | 14 (58.33) | 15 (55.56) | 13 (54.17) | 18 (69.23) | 14 (48.28) | 0.76 |
| Operating room | 4 (16.67) | 5 (18.52) | 3 (12.50) | 3 (11.54) | 7 (24.14) | |
| Hospitalization | 4 (16.67) | 7 (25.93) | 8 (33.33) | 5 (19.23) | 7 (24.14) | |
| Diagnoses at admission, n (%) | ||||||
| Cardiovascular | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (7.69) | 2 (6.90) | 0.82 |
| Respiratory | 6 (25.00) | 6 (22.22) | 7 (29.17) | 4 (15.38) | 5 (17.24) | |
| Gastrointestinal | 9 (37.50) | 5 (18.52) | 4 (16.67) | 5 (19.23) | 6 (20.69) | |
| Neurological | 1 (4.17) | 1 (3.70) | 1 (4.17) | 2 (7.69) | 2 (6.90) | |
| Sepsis | 6 (25.00) | 1 (3.70) | 7 (29.17) | 9 (34.62) | 12 (41.38) | |
| Trauma | 0 (0.00) | 1 (3.70) | 1 (4.17) | 0 (0.00) | 0 (0.00) | |
| Metabolic | 1 (4.17) | 1 (3.70) | 0 (0.00) | 0 (0.00) | 1 (3.45) | |
| Hematologic | 0 (0.00) | 2 (7.41) | 0 (0.00) | 1 (3.85) | 1 (3.45) | |
| Renal/Genitourinary | 0 (0.00) | 1 (3.70) | 3 (12.50) | 3 (11.54) | 0 (0.00) | |
| Site of infection, n (%) | ||||||
| Pulmonary | 7 (29.17) | 11 (40.74) | 9 (39.13) | 11 (42.31) | 10 (34.48) | 0.83 |
| Gastrointestinal | 10 (41.67) | 8 (29.63) | 5 (21.74) | 5 (19.23) | 11 (37.93) | |
| Nefrourinary | 3 (12.50) | 3 (11.11) | 6 (26.09) | 6 (23.08) | 3 (10.34) | |
| CNS | 0 (0.00) | 2 (7.41) | 0 (0.00) | 0 (0.00) | 1 (3.45) | |
| Skin and soft tissues | 2 (8.33) | 2 (7.41) | 2 (8.70) | 2 (7.69) | 2 (6.90) | |
| Endocarditis | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (3.45) | |
| Gastrointestinal | 0 (0.00) | 1 (3.70) | 0 (0.00) | 2 (7.69) | 1 (3.45) | |
| Proinflammatory | Anti-Inflammatory | |||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | |||
| Group | IL-1β | IL-1β | p | IL-4 | IL-4 | p |
| Vit C | 9.8 (8.12–5700) | 8.1 (6.4–178.79) | 0.16 | 3.3 (0.39–328) | 2.2 (0.39–65.2) | 0.53 |
| Vit E | 8.1 (8.1–360) | 8.1 (8.1–170.7) | 0.66 | 5.35 (0.39–275.5) | 13.7 (0.39–484.9) | 0.21 |
| NAC | 10.8 (8.1–139.5) | 8.1 (8.1–172.7) | 0.27 | 4.05 (0.39–4262.7) | 6.4 (0.39–844.4) | 0.39 |
| MT | 8.1 (8.1–691) | 8.1 (7.1–1050) | 0.34 | 3.8 (0.39–184) | 2.09 (0.39–117) | 0.04 |
| Control | 8.1 (8.1–4110) | 8.2 (8.1–1337) | 0.28 | 3.7 (0.39–1307.7) | 1.5 (0.39–1000) | 0.52 |
| IL-6 | IL-6 | IL-10 | IL-10 | p | ||
| Vit C | 124.89 (22.35–546.49) | 59.82 (14.93–148.1) | 0.006 | 73.3 (2.97–1094.30) | 66 (2.97–2762.5) | 0.56 |
| Vit E | 106.345 (5.64–512.885) | 16.965 (0.94–233.24) | 0.07 | 106.3 (2.9–1322) | 74 (2.9–362.9) | 0.17 |
| NAC | 127.48 (31.27–997.595) | 69.85 (3.805–231.895) | 0.90 | 56.5 (4.6–1007.97) | 54.7 (7.34–1370.7) | 0.88 |
| MT | 344.87 (15.13–564.88) | 23.25 (7.45–238.45) | 0.001 | 70.5 (6.7–1815) | 57.4 (4.1–5.6) | 0.11 |
| Control | 236.24 (22.92–2197.01) | 35.97 (6.91–112.95) | 0.002 | 107 (2.97–1683) | 55.1 (1.9–3960) | 0.08 |
| IL-8 | IL-8 | p | TGF-β | TGF-β | p | |
| Vit C | 12.6 (1.45–83.6) | 6.1 (1.45–46.34) | 0.14 | 44.7 (8.8–414.2) | 46.9 (0–535.2) | 0.80 |
| Vit E | 19.05 (1.45–241) | 8.6 (1.45–269) | 0.16 | 63 (8.8–452.8) | 52.9 (0–826.7) | 0.41 |
| NAC | 14.8 (1.45–283) | 9.5 (1.84–269.9) | 0.73 | 27.6 (8.8–411.6) | 52.9 (8.8–789.7) | 0.08 |
| MT | 13.8 (1.45–227.17) | 8.9 (1.45–367) | 0.001 | 45.09 (8.8–616.3) | 51.4 (8.8–359.06) | 0.53 |
| Control | 21.9 (1.45–688.8) | 7.6 (1.45–100) | 0.001 | 45.1 (8.8–1033) | 75.8 (8.8–356.9) | 0.67 |
| Proinflammatory | Regulatory cytokines | |||||
| TNF-α | TNF-α | IL-2 | IL-2 | p | ||
| Vit C | 0.80 (0.41–99.6) | 0.65 (0.41–18.9) | 0.26 | 1.4 (0.45–100.4) | 0.45 (0.45–10.6) | 0.36 |
| Vit E | 0.74 (0.41–119) | 0.8 (0.41–144.9) | 0.85 | 0.56 (0.30–51.4) | 1.13 (0.45–102.7) | 0.01 |
| NAC | 1.06 (0.41–76.6) | 0.1 (0.41–144.9) | 0.04 | 0.58 (0.45–51.4) | 0.66 (0.45–102.7) | 0.15 |
| MT | 0.64 (0.41–20.4) | 0.80 (0.41–49.6) | 0.84 | 0.45 (0.45–8.9) | 0.45 (0.45–18.4) | 0.34 |
| Control | 0.41 (0.41–32.6) | 0.54 (0.41–65) | 0.79 | 0.51 (0.45–3.80) | 0.45 (0.45–15.3) | 0.54 |
| IP-10 | IP-10 | p | IL-12 | IL-12 | p | |
| Vit C | 73.3 (2.97–1094.3) | 66.05 (2.97–2762.53) | 0.57 | 1.2 (0.85–21.8) | 1.06 (0.85–32.1) | 0.12 |
| Vit E | 106.3 (2.97–1322.2) | 74.5 (2.9–362.9) | 0.17 | 1.49 (0.85–176.4) | 1.45 (0.85–222.7) | 0.01 |
| NAC | 56.5 (4.68–1007.9) | 54.7 (7.34–1370.7) | 0.88 | 1.49 (0.85–62.36) | 1.4 (0.85–187.02) | 0.04 |
| MT | 69.6 (6.7–1001.7) | 57.4 (4.1–565.8) | 0.11 | 1.1 (0.85–27.8) | 0.92 (0.85–49.1) | 0.11 |
| Control | 107.6 (2.9–1683.8) | 55.1 (1.9–3960.96) | 0.08 | 1.2 (0.85–46.7) | 1.2 (0.85–59.4) | 0.75 |
| MCP-1 | MCP-1 | p | IFN-γ | IFN-γ | p | |
| Vit C | 324 (15.1–4321) | 216 (5.3–1641) | 0.11 | 3.08 (3.05–31.40) | 3.05 (3.05–23.01) | 0.46 |
| Vit E | 189.1 (1.6–2700) | 119 (1.6–2866) | 0.45 | 3.15 (3.05–20.77) | 3.05 (3.05–96.16) | 0.03 |
| NAC | 257.6 (78.08–2954.7) | 152.8 (23.5–2569.9) | 0.44 | 3.49 (3.05–23.98) | 3.93 (3.05–36.71) | 0.15 |
| MT | 295 (23.2–3479) | 210.7 (31.2–2504.4) | 0.01 | 3.05 (3.05–18.18) | 3.05 (3.05–14.09) | 0.53 |
| Control | 316 (38–4110) | 185. (11.03–1000) | 0.002 | 3.2 (3.05–67.44) | 3.05 (3.05–33.24) | 0.21 |
| Variable | Level | Variable | Coef. | Std. Err. | t | p-Value | IC95% | Corr. | |
|---|---|---|---|---|---|---|---|---|---|
| u1 | High | PCT | 0.0172182 | 0.0042318 | 4.07 | 0.0001 | 0.0085873 | 0.0258491 | 0.9529 |
| Carbonyl | −0.0068545 | 0.0030781 | −2.23 | 0.033 | −0.0131324 | −0.0005766 | |||
| Low | TAC | −0.0002907 | 0.0000967 | −3.01 | 0.005 | −0.000488 | −0.0000935 | ||
| GSH | 5.295229 | 1.522535 | 3.48 | 0.002 | 2.189998 | 8.400459 | |||
| Vit C | 6.144225 | 0.6809717 | 9.02 | 0.001 | 4.755374 | 7.533075 | |||
| v1 | Low | IL4 | −0.0073968 | 0.0025368 | −2.92 | 0.007 | −0.0125706 | −0.002223 | |
| High | IP10 | 0.0009865 | 0.0001994 | 4.95 | 0.001 | 0.0005798 | 0.0013932 | ||
| IL1B | 0.0002558 | 0.000123 | 2.08 | 0.046 | 5.01 × 10−6 | 0.0005067 | |||
| MCP 1 | −0.0006084 | 0.0001506 | −4.04 | 0.001 | −0.0009155 | −0.0003012 | |||
| IL6 | 0.0003399 | 0.0000436 | 7.8 | 0.001 | 0.0002511 | 0.0004288 | |||
| ILl17a | −0.0098983 | 0.0025994 | −3.81 | 0.001 | −0.0151998 | −0.0045967 | |||
| TNFα | 0.049159 | 0.0144382 | 3.4 | 0.002 | 0.0197122 | 0.0786059 | |||
| u2 | High | LPO | −0.1764843 | 0.0744333 | −2.37 | 0.024 | −0.328292 | −0.0246766 | 0.8643 |
| Carbonyl | 0.0164766 | 0.0056307 | 2.93 | 0.006 | 0.0049928 | 0.0279604 | |||
| Low | GSH | −11.67688 | 2.785091 | −4.19 | 0.001 | −17.35711 | −5.996647 | ||
| Selenium | 492.1174 | 139.0012 | 3.54 | 0.001 | 208.6226 | 775.6122 | |||
| Thiol | −0.1522415 | 0.0385513 | −3.95 | 0.001 | −0.2308675 | −0.0736155 | |||
| v2 | Low | il4 | 0.0124842 | 0.0046404 | 2.69 | 0.011 | 0.00302 | 0.0219484 | |
| IL12p70 | 0.0367106 | 0.0155704 | 2.36 | 0.025 | 0.0049544 | 0.0684667 | |||
| IFNy | −0.0140323 | 0.0035662 | −3.93 | 0.001 | −0.0213055 | −0.006759 | |||
| TGFB1 | 0.0031482 | 0.00154 | 2.04 | 0.049 | 7.40 × 10−6 | 0.006289 | |||
| High | IL1B | −0.0007156 | 0.000225 | −3.18 | 0.003 | −0.0011744 | −0.0002568 | ||
| MCP1 | 0.0013014 | 0.0002755 | 4.72 | 0.001 | 0.0007396 | 0.0018632 | |||
| IL6a | 0.0002323 | 0.0000797 | 2.92 | 0.007 | 0.0000698 | 0.0003947 | |||
| u3 | High | SOFA | −0.3846204 | 0.1060993 | −3.63 | 0.001 | −0.6010113 | −0.1682295 | 0.8218 |
| PCT | −0.0219292 | 0.009223 | −2.38 | 0.024 | −0.0407397 | −0.0031188 | |||
| PCR | 0.0441247 | 0.0167844 | 2.63 | 0.013 | 0.0098926 | 0.0783568 | |||
| Low | GSH | 6.547262 | 3.318249 | 1.97 | 0.057 | −0.2203513 | 13.31488 | ||
| GSHpx | −6.29239 | 1.747836 | −3.6 | 0.001 | −9.857125 | −2.727655 | |||
| v3 | Low | IL4 | 0.0070789 | 0.0055288 | 1.28 | 0.21 | −0.004197 | 0.0183549 | |
| TGFB1 | 0.0038936 | 0.0018348 | 2.12 | 0.042 | 0.0001515 | 0.0076356 | |||
| IL12p70 | −0.0400625 | 0.0185511 | −2.16 | 0.039 | −0.0778978 | −0.0022272 | |||
| High | IL1B | −0.0007372 | 0.000268 | −2.75 | 0.01 | −0.0012838 | −0.0001905 | ||
| IL8 | 0.0300677 | 0.0085392 | 3.52 | 0.001 | 0.0126518 | 0.0474836 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Torres, I.; Aisa-Álvarez, A.; Casarez-Alvarado, S.; Borrayo, G.; Márquez-Velasco, R.; Guarner-Lans, V.; Manzano-Pech, L.; Cruz-Soto, R.; Gonzalez-Marcos, O.; Fuentevilla-Álvarez, G.; et al. Impact of Treatment with Antioxidants as an Adjuvant to Standard Therapy in Patients with Septic Shock: Analysis of the Correlation between Cytokine Storm and Oxidative Stress and Therapeutic Effects. Int. J. Mol. Sci. 2023, 24, 16610. https://doi.org/10.3390/ijms242316610
Pérez-Torres I, Aisa-Álvarez A, Casarez-Alvarado S, Borrayo G, Márquez-Velasco R, Guarner-Lans V, Manzano-Pech L, Cruz-Soto R, Gonzalez-Marcos O, Fuentevilla-Álvarez G, et al. Impact of Treatment with Antioxidants as an Adjuvant to Standard Therapy in Patients with Septic Shock: Analysis of the Correlation between Cytokine Storm and Oxidative Stress and Therapeutic Effects. International Journal of Molecular Sciences. 2023; 24(23):16610. https://doi.org/10.3390/ijms242316610
Chicago/Turabian StylePérez-Torres, Israel, Alfredo Aisa-Álvarez, Sergio Casarez-Alvarado, Gabriela Borrayo, Ricardo Márquez-Velasco, Verónica Guarner-Lans, Linaloe Manzano-Pech, Randall Cruz-Soto, Omar Gonzalez-Marcos, Giovanny Fuentevilla-Álvarez, and et al. 2023. "Impact of Treatment with Antioxidants as an Adjuvant to Standard Therapy in Patients with Septic Shock: Analysis of the Correlation between Cytokine Storm and Oxidative Stress and Therapeutic Effects" International Journal of Molecular Sciences 24, no. 23: 16610. https://doi.org/10.3390/ijms242316610
APA StylePérez-Torres, I., Aisa-Álvarez, A., Casarez-Alvarado, S., Borrayo, G., Márquez-Velasco, R., Guarner-Lans, V., Manzano-Pech, L., Cruz-Soto, R., Gonzalez-Marcos, O., Fuentevilla-Álvarez, G., Gamboa, R., Saucedo-Orozco, H., Franco-Granillo, J., & Soto, M. E. (2023). Impact of Treatment with Antioxidants as an Adjuvant to Standard Therapy in Patients with Septic Shock: Analysis of the Correlation between Cytokine Storm and Oxidative Stress and Therapeutic Effects. International Journal of Molecular Sciences, 24(23), 16610. https://doi.org/10.3390/ijms242316610