PARIN5, a Novel Thrombin Receptor Antagonist Modulates a Streptozotocin Mice Model for Diabetic Encephalopathy
Abstract
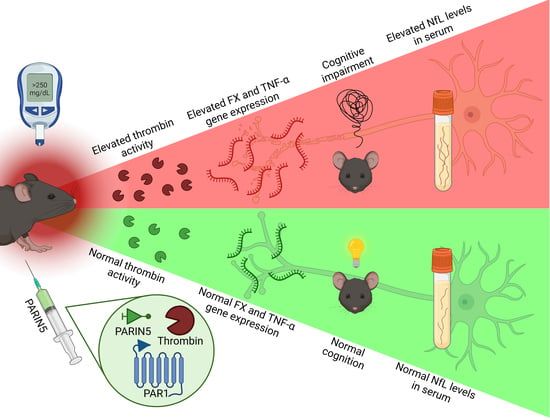
:1. Introduction
2. Results
2.1. Body Weight and Glucose Levels
2.2. Serum NfL Levels
2.3. Thrombin Activity
2.4. Behavioral Studies—Reduced Rearing Behavior on Open Field Test
2.5. Novel Object Recognition
2.6. Brain Gene Expression of TNF-α, FX, Prothrombin, and PAR1
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Diabetes Induction
4.3. Treatment Protocol
4.4. Neurofilament Light Chain (NfL)
4.5. Thrombin Activity Assay
4.6. Behavioral Studies
4.6.1. Open Field
4.6.2. Novel Object Recognition
4.7. Real-Time Polymerase Chain Reaction (RT-PCR)
4.8. Statistics
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forouhi, N.G.; Wareham, N.J. Epidemiology of Diabetes. Medicine 2019, 47, 22–27. [Google Scholar] [CrossRef]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic Neuropathy. Nat. Rev. Dis. Prim. 2019, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Mijnhout, G.S.; Scheltens, P.; Diamant, M.; Biessels, G.J.; Wessels, A.M.; Simsek, S.; Snoek, F.J.; Heine, R.J. Diabetic Encephalopathy: A Concept in Need of a Definition. Diabetologia 2006, 49, 1447–1448. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Shi, J.; Yin, Q.; Li, X.; Sheng, Y.; Han, J.; Zhuang, P.; Zhang, Y. Morphological and Pathological Characteristics of Brain in Diabetic Encephalopathy. J. Alzheimer’s Dis. 2018, 65, 15–28. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H. Inflammation and Thrombosis: Roles of Neutrophils, Platelets and Endothelial Cells and Their Interactions in Thrombus Formation during Sepsis. J. Thromb. Haemost. 2018, 16, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Gofrit, S.; Shavit-Stein, E. The Neuro-Glial Coagulonome: The Thrombin Receptor and Coagulation Pathways as Major Players in Neurological Diseases. Neural Regen. Res. 2019, 14, 2043–2053. [Google Scholar] [PubMed]
- Rom, S.; Zuluaga-Ramirez, V.; Gajghate, S.; Seliga, A.; Winfield, M.; Heldt, N.A.; Kolpakov, M.A.; Bashkirova, Y.V.; Sabri, A.K.; Persidsky, Y. Hyperglycemia-Driven Neuroinflammation Compromises BBB Leading to Memory Loss in Both Diabetes Mellitus (DM) Type 1 and Type 2 Mouse Models. Mol. Neurobiol. 2019, 56, 1883–1896. [Google Scholar] [CrossRef] [PubMed]
- Vittal Rao, H.; Bihaqi, S.W.; Iannucci, J.; Sen, A.; Grammas, P.; Rhea, E. Thrombin Signaling Contributes to High Glucose-Induced Injury of Human Brain Microvascular Endothelial Cells. J. Alzheimer’s Dis. 2021, 79, 211–224. [Google Scholar] [CrossRef]
- Tiruppathi, C.; Naqvi, T.; Sandoval, R.; Mehta, D.; Malik, A.B. Synergistic Effects of Tumor Necrosis Factor-α and Thrombin in Increasing Endothelial Permeability. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2001, 281, L958–L968. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.; Reiser, G. Protease-Activated Receptors in the Brain: Receptor Expression, Activation, and Functions in Neurodegeneration and Neuroprotection. Brain Res. Rev. 2007, 56, 331–345. [Google Scholar] [CrossRef]
- Petzold, T.; Thienel, M.; Dannenberg, L.; Mourikis, P.; Helten, C.; Ayhan, A.; M’Pembele, R.; Achilles, A.; Trojovky, K.; Konsek, D.; et al. Rivaroxaban Reduces Arterial Thrombosis by Inhibition of FXa-Driven Platelet Activation via Protease Activated Receptor-1. Circ. Res. 2020, 126, 486–500. [Google Scholar] [CrossRef] [PubMed]
- Syrovets, T.; Lunov, O.; Simmet, T. Plasmin as a Proinflammatory Cell Activator. J. Leukoc. Biol. 2012, 92, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Esmon, C.T. Protein C Anticoagulant System—Anti-Inflammatory Effects. Semin. Immunopathol. 2012, 34, 127–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, K.M.; Covic, L.; Kuliopulos, A. Matrix Metalloproteases and PAR1 Activation. Blood 2013, 121, 431. [Google Scholar] [CrossRef] [Green Version]
- Sinha, R.K.; Wang, Y.; Zhao, Z.; Xu, X.; Burnier, L.; Gupta, N.; Fernández, J.A.; Martin, G.; Kupriyanov, S.; Mosnier, L.O.; et al. PAR1 Biased Signaling Is Required for Activated Protein C in Vivo Benefits in Sepsis and Stroke. Blood 2018, 131, 1163–1171. [Google Scholar] [CrossRef]
- Bushi, D.; Ben Shimon, M.; Shavit Stein, E.; Chapman, J.; Maggio, N.; Tanne, D. Increased Thrombin Activity Following Reperfusion after Ischemic Stroke Alters Synaptic Transmission in the Hippocampus. J. Neurochem. 2015, 135, 1140–1148. [Google Scholar] [CrossRef] [Green Version]
- Shavit-Stein, E.; Aronovich, R.; Sylantiev, C.; Gera, O.; Gofrit, S.G.; Chapman, J.; Dori, A. Blocking Thrombin Significantly Ameliorates Experimental Autoimmune Neuritis. Front. Neurol. 2018, 9, 1139. [Google Scholar] [CrossRef] [Green Version]
- Shavit-Stein, E.; Sheinberg, E.; Golderman, V.; Sharabi, S.; Wohl, A.; Gofrit, S.G.; Zivli, Z.; Shelestovich, N.; Last, D.; Guez, D.; et al. A Novel Compound Targeting Protease Receptor 1 Activators for the Treatment of Glioblastoma. Front. Neurol. 2018, 9, 1087. [Google Scholar] [CrossRef] [Green Version]
- Shavit-stein, E.; Gofrit, S.G.; Gayster, A.; Teldan, Y.; Ron, A. Treatment of Diabetic Neuropathy with A Novel PAR1-Targeting Molecule. Biomolecules 2020, 10, 1552. [Google Scholar] [CrossRef]
- Itzekson, Z.; Maggio, N.; Milman, A.; Shavit, E.; Pick, C.G.; Chapman, J. Reversal of Trauma-Induced Amnesia in Mice by a Thrombin Receptor Antagonist. J. Mol. Neurosci. 2014, 53, 87–95. [Google Scholar] [CrossRef]
- De Luca, C.; Colangelo, A.M.; Alberghina, L.; Papa, M. Neuro-Immune Hemostasis: Homeostasis and Diseases in the Central Nervous System. Front. Cell. Neurosci. 2018, 12, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shavit-Stein, E.; Aronovich, R.; Sylantiev, C.; Gofrit, S.G.; Chapman, J.; Dori, A. The Role of Thrombin in the Pathogenesis of Diabetic Neuropathy. PLoS ONE 2019, 14, e0219453. [Google Scholar] [CrossRef] [PubMed]
- Virtuoso, A.; Colangelo, A.M.; Korai, S.A.; Izzo, S.; Todisco, A.; Giovannoni, R.; Lavitrano, M.; Papa, M.; Cirillo, G. Inhibition of Plasminogen/Plasmin System Retrieves Endogenous Nerve Growth Factor and Adaptive Spinal Synaptic Plasticity Following Peripheral Nerve Injury. Neurochem. Int. 2021, 148, 105113. [Google Scholar] [CrossRef] [PubMed]
- Machida, T.; Takata, F.; Matsumoto, J.; Miyamura, T.; Hirata, R.; Kimura, I.; Kataoka, Y.; Dohgu, S.; Yamauchi, A. Contribution of Thrombin-Reactive Brain Pericytes to Blood-Brain Barrier Dysfunction in an in Vivo Mouse Model of Obesity-Associated Diabetes and an in Vitro Rat Model. PLoS ONE 2017, 12, e0177447. [Google Scholar] [CrossRef] [Green Version]
- Machida, T.; Dohgu, S.; Takata, F.; Matsumoto, J.; Kimura, I.; Koga, M.; Nakamoto, K.; Yamauchi, A.; Kataoka, Y. Role of Thrombin-PAR1-PKCθ/δ Axis in Brain Pericytes in Thrombin-Induced MMP-9 Production and Blood-Brain Barrier Dysfunction in Vitro. Neuroscience 2017, 350, 146–157. [Google Scholar] [CrossRef]
- Golderman, V.; Berkowitz, S.; Gofrit, S.G.; Gera, O.; Aharoni, S.A.; Zohar, D.N.; Keren, D.; Dori, A.; Chapman, J.; Shavit-Stein, E. Thrombin Activity in Rodent and Human Skin: Modified by Inflammation and Correlates with Innervation. Biomedicines 2022, 10, 1461. [Google Scholar] [CrossRef]
- Itsekson-Hayosh, Z.; Shavit-Stein, E.; Katzav, A.; Rubovitch, V.; Maggio, N.; Chapman, J.; Harnof, S.; Pick, C.G.C.G. Minimal Traumatic Brain Injury in Mice: Protease-Activated Receptor 1 and Thrombin-Related Changes. J. Neurotrauma 2016, 1854, 1848. [Google Scholar] [CrossRef]
- Davalos, D.; Baeten, K.M.; Whitney, M.A.; Mullins, E.S.; Friedman, B.; Olson, E.S.; Ryu, J.K.; Smirnoff, D.S.; Petersen, M.A.; Bedard, C.; et al. Early Detection of Thrombin Activity in Neuroinflammatory Disease. Ann. Neurol. 2014, 75, 303–308. [Google Scholar] [CrossRef] [Green Version]
- Itsekson-Hayosh, Z.; Shavit-Stein, E.; Last, D.; Goez, D.; Daniels, D.; Bushi, D.; Gera, O.; Zibly, Z.; Mardor, Y.; Chapman, J.; et al. Thrombin Activity and Thrombin Receptor in Rat Glioblastoma Model: Possible Markers and Targets for Intervention? J. Mol. Neurosci. 2015, 56, 644–651. [Google Scholar] [CrossRef]
- Ben Shimon, M.; Shavit-Stein, E.; Altman, K.; Pick, C.G.; Maggio, N. Thrombin as Key Mediator of Seizure Development Following Traumatic Brain Injury. Front. Pharmacol. 2020, 10, 1532. [Google Scholar] [CrossRef]
- Gisslén, M.; Price, R.W.; Andreasson, U.; Norgren, N.; Nilsson, S.; Hagberg, L.; Fuchs, D.; Spudich, S.; Blennow, K.; Zetterberg, H. Plasma Concentration of the Neurofilament Light Protein (NFL) Is a Biomarker of CNS Injury in HIV Infection: A Cross-Sectional Study. EBioMedicine 2016, 3, 135–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as Biomarkers in Neurological Disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Bjornevik, K.; Munger, K.L.; Cortese, M.; Barro, C.; Healy, B.C.; Niebuhr, D.W.; Scher, A.I.; Kuhle, J.; Ascherio, A. Serum Neurofilament Light Chain Levels in Patients with Presymptomatic Multiple Sclerosis. JAMA Neurol. 2020, 77, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Hepner, A.; Porter, J.; Hare, F.; Nasir, S.S.; Zetterberg, H.; Blennow, K.; Martin, M.G. Serum Neurofilament Light, Glial Fibrillary Acidic Protein and Tau Are Possible Serum Biomarkers for Activity of Brain Metastases and Gliomas. World J. Oncol. 2019, 10, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bittner, S.; Oh, J.; Havrdová, E.K.; Tintoré, M.; Zipp, F. The Potential of Serum Neurofilament as Biomarker for Multiple Sclerosis. Brain 2021, 144, 2954–2963. [Google Scholar] [CrossRef]
- Engel, S.; Boedecker, S.; Marczynski, P.; Bittner, S.; Steffen, F.; Weinmann, A.; Schwarting, A.; Zipp, F.; Weinmann-Menke, J.; Luessi, F. Association of Serum Neurofilament Light Chain Levels and Neuropsychiatric Manifestations in Systemic Lupus Erythematosus. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211051496. [Google Scholar] [CrossRef]
- Marutani, N.; Akamine, S.; Kanayama, D.; Gotoh, S.; Yanagida, K.; Maruyama, R.; Mori, K.; Miyamoto, T.; Adachi, H.; Sakagami, Y.; et al. Plasma NfL Is Associated with Mild Cognitive Decline in Patients with Diabetes. Psychogeriatrics 2022, 22, 353–359. [Google Scholar] [CrossRef]
- Meregalli, C.; Fumagalli, G.; Alberti, P.; Canta, A.; Carozzi, V.A.; Chiorazzi, A.; Monza, L.; Pozzi, E.; Sandelius, Å.; Blennow, K.; et al. Neurofilament Light Chain as Disease Biomarker in a Rodent Model of Chemotherapy Induced Peripheral Neuropathy. Exp. Neurol. 2018, 307, 129–132. [Google Scholar] [CrossRef] [Green Version]
- Mariotto, S.; Farinazzo, A.; Magliozzi, R.; Alberti, D.; Monaco, S.; Ferrari, S. Serum and Cerebrospinal Neurofilament Light Chain Levels in Patients with Acquired Peripheral Neuropathies. J. Peripher. Nerv. Syst. 2018, 23, 174–177. [Google Scholar] [CrossRef]
- Aharoni, R.; Eilam, R.; Lerner, S.; Shavit-Stein, E.; Dori, A.; Chapman, J.; Arnon, R. Neuroprotective Effect of Glatiramer Acetate on Neurofilament Light Chain Leakage and Glutamate Excess in an Animal Model of Multiple Sclerosis. Int. J. Mol. Sci. 2021, 22, 13419. [Google Scholar] [CrossRef]
- Shavit-Stein, E.; Berkowitz, S.; Davidy, T.; Fennig, U.; Gofrit, S.G.; Dori, A.; Maggio, N. Modulation of the Thrombin Pathway Restores LTP in a Pilocarpine Mice Model of Status Epilepticus. Front. Cell. Neurosci. 2022, 16, 900925. [Google Scholar] [CrossRef] [PubMed]
- Varatharaj, A.; Galea, I. The Blood-Brain Barrier in Systemic Inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, J.; Wang, L.; Zhang, L.; Qin, C.; Song, Y.; Ma, Y.; Chen, Y.; Chen, S.; Wang, Y.; Zhang, Z.; et al. Blood-Brain Barrier Disruption Induced Cognitive Impairment Is Associated with Increase of Inflammatory Cytokine. Front. Aging Neurosci. 2018, 10, 129. [Google Scholar] [CrossRef] [Green Version]
- Arai, T.; Miklossy, J.; Klegeris, A.; Quo, J.P.; McGeer, P.L. Thrombin and Prothrombin Are Expressed by Neurons and Glial Cells and Accumulate in Neurofibrillary Tangles in Alzheimer Disease Brain. J. Neuropathol. Exp. Neurol. 2006, 65, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Zilliox, L.A.; Chadrasekaran, K.; Kwan, J.Y.; Russell, J.W. Diabetes and Cognitive Impairment. Curr. Diabetes Rep. 2016, 16, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturman, O.; Germain, P.L.; Bohacek, J. Exploratory Rearing: A Context- and Stress-Sensitive Behavior Recorded in the Open-Field Test. Stress 2018, 21, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Watt, C.; Sanchez-Rangel, E.; Hwang, J.J. Glycemic Variability and CNS Inflammation: Reviewing the Connection. Nutrients 2020, 12, 3906. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.R.; Parra-Izquierdo, I.; Gruber, A.; Shatzel, J.J.; Pham, P.; Sherman, L.S.; McCarty, O.J.T.; Verbout, N.G. Thrombin Generation and Activity in Multiple Sclerosis. Metab. Brain Dis. 2021, 36, 407–420. [Google Scholar] [CrossRef]
- Beilin, O.; Karussis, D.M.; Korczyn, A.D.; Gurwitz, D.; Aronovich, R.; Hantai, D.; Grigoriadis, N.; Mizrachi-Kol, R.; Chapman, J. Increased Thrombin Inhibition in Experimental Autoimmune Encephalomyelitis. J. Neurosci. Res. 2005, 79, 351–359. [Google Scholar] [CrossRef]
- Han, M.H.; Hwang, S.-I.; Roy, D.B.; Lundgren, D.H.; Price, J.V.; Ousman, S.S.; Fernald, G.H.; Gerlitz, B.; Robinson, W.H.; Baranzini, S.E.; et al. Proteomic Analysis of Active Multiple Sclerosis Lesions Reveals Therapeutic Targets. Nature 2008, 451, 1076–1081. [Google Scholar] [CrossRef]
- Kumar Datusalia, A.; Sunder Sharma, S. NF-ΚB Inhibition Resolves Cognitive Deficits in Experimental Type 2 Diabetes Mellitus through CREB and Glutamate/GABA Neurotransmitters Pathway. Curr. Neurovascular Res. 2016, 13, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, 5–47. [Google Scholar] [CrossRef] [PubMed]
- Bushi, D.; Chapman, J.; Katzav, A.; Shavit-Stein, E.; Molshatzki, N.; Maggio, N.; Tanne, D. Quantitative Detection of Thrombin Activity in an Ischemic Stroke Model. J. Mol. Neurosci. 2013, 51, 844–850. [Google Scholar] [CrossRef]
- Shavit-Stein, E.; Gerasimov, A.; Aharoni, S.; Gofrit, S.G.; Pikus, E.; Pick, C.G.; Maggio, N. Unexpected Role of Stress as a Possible Resilience Mechanism upon Mild Traumatic Brain Injury (MTBI) in Mice. Mol. Cell. Neurosci. 2021, 111, 103586. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Biala, G. The Novel Object Recognition Memory: Neurobiology, Test Procedure, and Its Modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object Recognition Test in Mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef]
- Shah, S.G.; Rashid, M.; Verma, T.; Ludbe, M.; Khade, B.; Gera, P.B.; Gupta, S. Establishing a Correlation between RIN and A260/280 along with the Multivariate Evaluation of Factors Affecting the Quality of RNA in Cryopreserved Cancer Bio-Specimen. Cell Tissue Bank. 2019, 20, 489–499. [Google Scholar] [CrossRef]






| Gene | Forward | Reverse |
|---|---|---|
| HPRT | GATTAGCGATGATGAACCAGGTT | CCTCCCATCTCCTTCATGACA |
| PAR1 | GCCTCCATCATGCTCATGAC | AAAGCAGACGATGAAGATGCA |
| FX | GTGGCCGGGAATGCAA | AACCCTTCATTGTCTTCGTTAATGA |
| TNF-α | AGATCAATCGGCCCGACTATCTC | GTTTGGGAAGGTTGGATGTTCGT |
| PT (prothrombin) | CCGAAAGGGCAACCTAGAGC | GGCCCAGAACACGTCTGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golderman, V.; Goldberg, Z.; Gofrit, S.G.; Dori, A.; Maggio, N.; Chapman, J.; Sher, I.; Rotenstreich, Y.; Shavit-Stein, E. PARIN5, a Novel Thrombin Receptor Antagonist Modulates a Streptozotocin Mice Model for Diabetic Encephalopathy. Int. J. Mol. Sci. 2023, 24, 2021. https://doi.org/10.3390/ijms24032021
Golderman V, Goldberg Z, Gofrit SG, Dori A, Maggio N, Chapman J, Sher I, Rotenstreich Y, Shavit-Stein E. PARIN5, a Novel Thrombin Receptor Antagonist Modulates a Streptozotocin Mice Model for Diabetic Encephalopathy. International Journal of Molecular Sciences. 2023; 24(3):2021. https://doi.org/10.3390/ijms24032021
Chicago/Turabian StyleGolderman, Valery, Zehavit Goldberg, Shany Guly Gofrit, Amir Dori, Nicola Maggio, Joab Chapman, Ifat Sher, Ygal Rotenstreich, and Efrat Shavit-Stein. 2023. "PARIN5, a Novel Thrombin Receptor Antagonist Modulates a Streptozotocin Mice Model for Diabetic Encephalopathy" International Journal of Molecular Sciences 24, no. 3: 2021. https://doi.org/10.3390/ijms24032021
APA StyleGolderman, V., Goldberg, Z., Gofrit, S. G., Dori, A., Maggio, N., Chapman, J., Sher, I., Rotenstreich, Y., & Shavit-Stein, E. (2023). PARIN5, a Novel Thrombin Receptor Antagonist Modulates a Streptozotocin Mice Model for Diabetic Encephalopathy. International Journal of Molecular Sciences, 24(3), 2021. https://doi.org/10.3390/ijms24032021