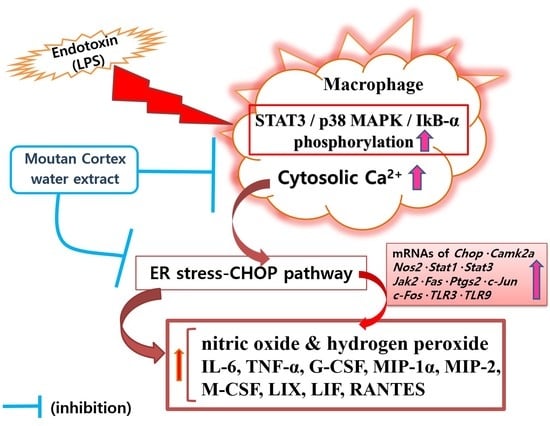
Moutan Cortex Extract Modulates Macrophage Activation via Lipopolysaccharide-Induced Calcium Signaling and ER Stress-CHOP Pathway
Abstract
:1. Introduction
2. Results
2.1. Extraction Yield and Total Flavonoid Content of CP
2.2. Cell Viability
2.3. NO Level from RAW 264.7
2.4. Cytosolic Calcium Level
2.5. Hydrogen Peroxide Level in RAW 264.7
2.6. Cytokines Level
2.7. Transcript Level of Inflammatory Gene Related to ER Stress
2.8. Activation of STAT3, p38 MAPK, and IkB-α
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of CP
4.3. The Total Flavonoid Content (TFC) of CP
4.4. Effect of CP on Cell Viability
4.5. Effect of CP on Level of NO, Cytosolic Ca2+, and Hydrogen Peroxide
4.6. Effect of CP on Cytokine Production
4.7. Effect of CP on Transcript Level of Inflammatory Genes
4.8. Effect of CP on Phosphorylation of STAT3, p38 MAPK, and IκB-α
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, G.; Zhang, L.; Zhu, Y. Determination of Glycosides and Sugars in Moutan Cortex by Capillary Electrophoresis with Electrochemical Detection. J. Pharm. Biomed. Anal. 2006, 41, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Gu, Z. Screening of Bioactive Compounds from Moutan Cortex and their Anti-Inflammatory Activities in Rat Synoviocytes. Evid Based. Complement. Alternat Med. 2009, 6, 57–63. [Google Scholar]
- Wang, Z.; He, C.; Peng, Y.; Chen, F.; Xiao, P. Origins, Phytochemistry, Pharmacology, Analytical Methods and Safety of Cortex Moutan (Paeonia Suffruticosa Andrew): A Systematic Review. Molecules 2017, 22, 946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, P.K.; Yang, C.Y.; Tsai, T.H.; Hsieh, C.L. Moutan Cortex Radicis Improves Lipopolysaccharide-Induced Acute Lung Injury in Rats through Anti-Inflammation. Phytomedicine 2012, 19, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.F.; Hsu, J.T.; Wu, K.C.; Hsiao, C.F.; Lin, J.A.; Cheng, Y.H.; Liu, Y.H.; Lee, D.Y.; Chang, H.H.; Cho, D.Y.; et al. A Systematic Identification of Anti-Inflammatory Active Components Derived from Mu Dan Pi and their Applications in Inflammatory Bowel Disease. Sci. Rep. 2020, 10, 17238. [Google Scholar] [CrossRef]
- Tabas, I.; Seimon, T.; Timmins, J.; Li, G.; Lim, W. Macrophage Apoptosis in Advanced Atherosclerosis. Ann. N. Y. Acad. Sci. 2009, 1173 (Suppl. S1), E40–E45. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Hui, X.; Hoo, R.L.C.; Ye, D.; Chan, C.Y.C.; Feng, T.; Wang, Y.; Lam, K.S.L.; Xu, A. Adipocyte-Secreted Exosomal microRNA-34a Inhibits M2 Macrophage Polarization to Promote Obesity-Induced Adipose Inflammation. J. Clin. Investig. 2019, 129, 834–849. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.H.; Fu, Y.C.; Zhang, D.W.; Yin, K.; Tang, C.K. Foam Cells in Atherosclerosis. Clin. Chim. Acta 2013, 424, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Marciniak, S.J.; Yun, C.Y.; Oyadomari, S.; Novoa, I.; Zhang, Y.; Jungreis, R.; Nagata, K.; Harding, H.P.; Ron, D. CHOP Induces Death by Promoting Protein Synthesis and Oxidation in the Stressed Endoplasmic Reticulum. Genes Dev. 2004, 18, 3066–3077. [Google Scholar] [CrossRef] [Green Version]
- Lim, Y.; Choi, J.; Lee, J.; Choi, C.H.; Kim, H.; Song, C. Mycobacterium Tuberculosis 38-kDa Antigen Induces Endoplasmic Reticulum Stress-Mediated Apoptosis Via Toll-Like Receptor 2/4. Apoptosis 2015, 20, 358–370. [Google Scholar] [CrossRef]
- Xue, X.; Piao, J.H.; Nakajima, A.; Sakon-Komazawa, S.; Kojima, Y.; Mori, K.; Yagita, H.; Okumura, K.; Harding, H.; Nakano, H. Tumor Necrosis Factor Alpha (TNFalpha) Induces the Unfolded Protein Response (UPR) in a Reactive Oxygen Species (ROS)-Dependent Fashion, and the UPR Counteracts ROS Accumulation by TNFalpha. J. Biol. Chem. 2005, 280, 33917–33925. [Google Scholar] [CrossRef] [PubMed]
- Timmins, J.M.; Ozcan, L.; Seimon, T.A.; Li, G.; Malagelada, C.; Backs, J.; Backs, T.; Bassel-Duby, R.; Olson, E.N.; Anderson, M.E.; et al. Calcium/calmodulin-Dependent Protein Kinase II Links ER Stress with Fas and Mitochondrial Apoptosis Pathways. J. Clin. Investig. 2009, 119, 2925–2941. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Ye, X.; Chen, M.; Li, H.; Wang, Y.; Zhong, M.; Zhong, C.; Zeng, B.; Xu, L.; He, X.; et al. Inhibition of NLRP3 Inflammasome Activation and Pyroptosis in Macrophages by Taraxasterol is Associated with its Regulation on mTOR Signaling. Front. Immunol. 2021, 12, 632606. [Google Scholar] [CrossRef]
- Jorgensen, I.; Miao, E.A. Pyroptotic Cell Death Defends Against Intracellular Pathogens. Immunol. Rev. 2015, 265, 130–142. [Google Scholar] [CrossRef] [Green Version]
- Endo, M.; Mori, M.; Akira, S.; Gotoh, T. C/EBP Homologous Protein (CHOP) is Crucial for the Induction of Caspase-11 and the Pathogenesis of Lipopolysaccharide-Induced Inflammation. J. Immunol. 2006, 176, 6245–6253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, M. Regulation of Nitric Oxide Synthesis and Apoptosis by Arginase and Arginine Recycling. J. Nutr. 2007, 137, 1616S–1620S. [Google Scholar] [CrossRef] [Green Version]
- Muendlein, H.I.; Jetton, D.; Connolly, W.M.; Eidell, K.P.; Magri, Z.; Smirnova, I.; Poltorak, A. CFLIPL Protects Macrophages from LPS-Induced Pyroptosis via Inhibition of Complex II Formation. Science 2020, 367, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, S.; Reynoso, M.; Geddis, A.V.; Mitrophanov, A.Y.; Matheny, R.W.J. LPS-Stimulated NF-kappaB p65 Dynamic Response Marks the Initiation of TNF Expression and Transition to IL-10 Expression in RAW 264.7 Macrophages. Physiol. Rep. 2018, 6, e13914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhi, W.; Liu, Y.; Wang, X.; Zhang, H. Recent Advances of Traditional Chinese Medicine for the Prevention and Treatment of Atherosclerosis. J. Ethnopharmacol. 2023, 301, 115749. [Google Scholar] [CrossRef]
- Bai, M.; Liu, H.; Wang, S.; Shu, Q.; Xu, K.; Zhou, J.; Xiong, X.; Huang, R.; Deng, J.; Yin, Y.; et al. Dietary Moutan Cortex Radicis Improves Serum Antioxidant Capacity and Intestinal Immunity and Alters Colonic Microbiota in Weaned Piglets. Front. Nutr. 2021, 8, 679129. [Google Scholar] [CrossRef]
- Jang, M.H.; Kim, K.Y.; Song, P.H.; Baek, S.Y.; Seo, H.L.; Lee, E.H.; Lee, S.G.; Park, K.I.; Ahn, S.C.; Kim, S.C.; et al. Moutan Cortex Protects Hepatocytes Against Oxidative Injury through AMP-Activated Protein Kinase Pathway. Biol. Pharm. Bull. 2017, 40, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Nakano, Y.; Yatsuzuka, R.; Ono, R.; Kamei, C. Inhibitory Effects of Moutan Cortex on Immediate Allergic Reactions. Biol. Pharm. Bull. 2007, 30, 1707–1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Song, A.; Hu, W.; Dai, M. The Anti-Atherosclerotic Effect of Paeonol Against Vascular Smooth Muscle Cell Proliferation by Up-Regulation of Autophagy via the AMPK/mTOR Signaling Pathway. Front. Pharmacol. 2018, 8, 948. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Shen, G.; Zhao, W.; Wang, F.; Jiang, X.; Huang, D. Paeonol, the Main Active Principles of Paeonia Moutan, Ameliorates Alcoholic Steatohepatitis in Mice. J. Ethnopharmacol. 2010, 128, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Park, G.; Piao, Y.; Kang, M.S.; Pak, Y.K.; Hong, S.; Oh, M.S. Effects of the Root Bark of Paeonia Suffruticosa on Mitochondria-Mediated Neuroprotection in an MPTP-Induced Model of Parkinson’s Disease. Food Chem. Toxicol. 2014, 65, 293–300. [Google Scholar] [CrossRef]
- Thorp, E.; Li, G.; Seimon, T.A.; Kuriakose, G.; Ron, D.; Tabas, I. Reduced Apoptosis and Plaque Necrosis in Advanced Atherosclerotic Lesions of Apoe −/− and Ldlr −/− Mice Lacking CHOP. Cell Metab. 2009, 9, 474–481. [Google Scholar] [CrossRef] [Green Version]
- Seimon, T.A.; Obstfeld, A.; Moore, K.J.; Golenbock, D.T.; Tabas, I. Combinatorial Pattern Recognition Receptor Signaling Alters the Balance of Life and Death in Macrophages. Proc. Natl. Acad. Sci. USA 2006, 103, 19794–19799. [Google Scholar] [CrossRef] [Green Version]
- Lim, W.S.; Timmins, J.M.; Seimon, T.A.; Sadler, A.; Kolodgie, F.D.; Virmani, R.; Tabas, I. Signal Transducer and Activator of Transcription-1 is Critical for Apoptosis in Macrophages Subjected to Endoplasmic Reticulum Stress in Vitro and in Advanced Atherosclerotic Lesions in Vivo. Circulation 2008, 117, 940–951. [Google Scholar] [CrossRef] [Green Version]
- Joseph, S.K.; Hajnoczky, G. IP3 Receptors in Cell Survival and Apoptosis: Ca2+ Release and Beyond. Apoptosis 2007, 12, 951–968. [Google Scholar] [CrossRef] [Green Version]
- Deniaud, A.; Sharaf el dein, O.; Maillier, E.; Poncet, D.; Kroemer, G.; Lemaire, C.; Brenner, C. Endoplasmic Reticulum Stress Induces Calcium-Dependent Permeability Transition, Mitochondrial Outer Membrane Permeabilization and Apoptosis. Oncogene 2008, 27, 285–299. [Google Scholar] [CrossRef] [Green Version]
- Lencesova, L.; Krizanova, O. IP(3) Receptors, Stress and Apoptosis. Gen. Physiol. Biophys. 2012, 31, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Mongillo, M.; Chin, K.T.; Harding, H.; Ron, D.; Marks, A.R.; Tabas, I. Role of ERO1-Alpha-Mediated Stimulation of Inositol 1,4,5-Triphosphate Receptor Activity in Endoplasmic Reticulum Stress-Induced Apoptosis. J. Cell Biol. 2009, 186, 783–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, Y.; Wang, Z.; Chai, G.; Xiong, Y.; Li, B.; Zhang, H.; Xin, R.; Qian, X.; Tang, Z.; Wu, J.; et al. Dehydrocostus Lactone Suppresses LPS-Induced Acute Lung Injury and Macrophage Activation through NF-kappaB Signaling Pathway Mediated by p38 MAPK and Akt. Molecules 2019, 24, 1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, E.; Wang, T.; Wang, F.; Wang, T.; Sun, L.; Li, L.; Niu, S.; Zhang, J. FGF21 Protects Against Ox-LDL Induced Apoptosis through Suppressing CHOP Expression in THP1 Macrophage Derived Foam Cells. BMC Cardiovasc. Disord. 2015, 15, 80. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Ren, W.; Jiang, Z.; Zhu, L. Regulation of the NLRP3 Inflammasome and Macrophage Pyroptosis by the p38 MAPK Signaling Pathway in a Mouse Model of Acute Lung Injury. Mol. Med. Rep. 2018, 18, 4399–4409. [Google Scholar] [CrossRef] [Green Version]
- Cazanave, S.C.; Elmi, N.A.; Akazawa, Y.; Bronk, S.F.; Mott, J.L.; Gores, G.J. CHOP and AP-1 Cooperatively Mediate PUMA Expression during Lipoapoptosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G236–G243. [Google Scholar] [CrossRef] [Green Version]
- Meares, G.P.; Liu, Y.; Rajbhandari, R.; Qin, H.; Nozell, S.E.; Mobley, J.A.; Corbett, J.A.; Benveniste, E.N. PERK-Dependent Activation of JAK1 and STAT3 Contributes to Endoplasmic Reticulum Stress-Induced Inflammation. Mol. Cell. Biol. 2014, 34, 3911–3925. [Google Scholar] [CrossRef] [Green Version]
- Chun, S.; Jee, S.Y.; Lee, S.G.; Park, S.J.; Lee, J.R.; Kim, S.C. Anti-Inflammatory Activity of the Methanol Extract of Moutan Cortex in LPS-Activated Raw264.7 Cells. Evid. Based Complement. Alternat Med. 2007, 4, 327–333. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.; Manzoor, Z.; Koo, J.; Kim, J.; Byeon, S.; Yoo, E.; Kang, H.; Hyun, J.; Lee, N.; Koh, Y. 3-Hydroxy-4,7-Megastigmadien-9-One, Isolated from Ulva Pertusa, Attenuates TLR9-Mediated Inflammatory Response by Down-Regulating Mitogen-Activated Protein Kinase and NF-kappaB Pathways. Pharm. Biol. 2017, 55, 435–440. [Google Scholar] [CrossRef] [Green Version]
- An, H.; Lee, J.; Park, W. Baicalin Modulates Inflammatory Response of Macrophages Activated by LPS via Calcium-CHOP Pathway. Cells 2022, 11, 3076. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, J.Y.; Kim, Y.J.; Kim, H.J.; Park, W. Rubi Fructus Water Extract Alleviates LPS-Stimulated Macrophage Activation via an ER Stress-Induced Calcium/CHOP Signaling Pathway. Nutrients 2020, 12, 3577. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, D.H.; Park, W. Conioselinum tenuissimum Root Extract Modulates Macrophage Activation via the Calcium–STAT3 Pathway. Processes 2022, 10, 2238. [Google Scholar] [CrossRef]
| Treatment Group | Cell Viability (%) | ||
|---|---|---|---|
| Basal (media only) | 100.00 | ± | 4.52 |
| 25 µg/mL of CP | 151.57 | ± | 12.61 # |
| 50 µg/mL of CP | 151.29 | ± | 9.31 # |
| 100 µg/mL of CP | 147.64 | ± | 7.32 # |
| 200 µg/mL of CP | 150.49 | ± | 4.23 # |
| Inflammatory Factor | Basal (Media Only) | LPS (LPS Alone) | Concentration (µg/mL) of CP with Lipopolysaccharide (1 µg/mL) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 100 | 200 | |||||||||||||||
| Nitric Oxide (%) | 100.00 | ± | 2.92 | 201.98 | ± | 3.55 | 197.49 | ± | 2.94 ** | 194.58 | ± | 3.94 *** | 192.21 | ± | 4.00 *** | 188.64 | ± | 4.44 *** |
| Cytosolic Calcium (%) | 100.00 | ± | 1.90 | 104.49 | ± | 2.32 | 100.08 | ± | 1.71* | 99.69 | ± | 1.19 * | 98.79 | ± | 2.88 ** | 94.97 | ± | 3.49 *** |
| Hydrogen Peroxide (24 h) | 100.00 | ± | 7.23 | 212.26 | ± | 21.74 | 168.01 | ± | 15.19 *** | 135.49 | ± | 8.55 *** | 98.20 | ± | 9.30 *** | 86.31 | ± | 8.55 *** |
| Hydrogen Peroxide (48 h) | 100.00 | ± | 7.71 | 202.68 | ± | 19.97 | 163.80 | ± | 15.09 *** | 133.12 | ± | 6.41 *** | 98.99 | ± | 8.88 *** | 86.41 | ± | 7.84 *** |
| Hydrogen Peroxide (72 h) | 100.00 | ± | 8.88 | 212.74 | ± | 25.08 | 178.12 | ± | 16.68 ** | 148.81 | ± | 7.36 *** | 111.47 | ± | 8.52 *** | 96.68 | ± | 8.68 *** |
| IL-6 (pg/mL) | 40.00 | ± | 3.28 | 27,159.00 | ± | 230.53 | 25,932.00 | ± | 123.79 ** | 25,848.25 | ± | 457.21 * | 25,431.25 | ± | 694.80 * | 25,622.67 | ± | 170.93 ** |
| MCP-1 (pg/mL) | 38.83 | ± | 6.71 | 2951.50 | ± | 327.74 | 2608.50 | ± | 241.64 | 2556.17 | ± | 224.95 | 2387.33 | ± | 332.41 | 2417.00 | ± | 227.05 |
| TNF-α (pg/mL) | 201.17 | ± | 43.48 | 6647.88 | ± | 97.18 | 5642.83 | ± | 114.48 ** | 5952.38 | ± | 370.63 ** | 5729.13 | ± | 583.20 * | 6047.33 | ± | 93.11 * |
| G-CSF (pg/mL) | 175.67 | ± | 44.88 | 27,526.50 | ± | 150.23 | 25,875.50 | ± | 93.66 ** | 26,044.67 | ± | 261.50 ** | 26,004.00 | ± | 534.14 * | 25,951.00 | ± | 51.80 *** |
| GM-CSF (pg/mL) | 34.00 | ± | 7.21 | 17,425.00 | ± | 306.59 | 16083.67 | ± | 1145.00 | 15,891.67 | ± | 2463.69 | 13095.17 | ± | 1767.27 | 15,996.83 | ± | 841.74 * |
| IL-10 (pg/mL) | 25.67 | ± | 3.79 | 4529.33 | ± | 627.70 | 4674.00 | ± | 260.85 | 4829.33 | ± | 338.25 | 4659.83 | ± | 831.75 | 4841.67 | ± | 165.21 |
| LIF (pg/mL) | 38.50 | ± | 3.19 | 8497.50 | ± | 289.28 | 7748.75 | ± | 437.68* | 7859.63 | ± | 250.35 * | 7633.50 | ± | 1278.55 | 8127.13 | ± | 473.55 |
| LIX (pg/mL) | 554.25 | ± | 35.74 | 6535.25 | ± | 243.25 | 5992.83 | ± | 159.99 | 5966.83 | ± | 286.08 * | 5842.75 | ± | 444.35 * | 6108.50 | ± | 163.19 * |
| M-CSF (pg/mL) | 33.50 | ± | 0.50 | 39.33 | ± | 3.40 | 31.63 | ± | 3.35* | 31.00 | ± | 1.80 *** | 33.67 | ± | 2.25 *** | 33.75 | ± | 2.53 * |
| MIP-1α (pg/mL) | 3954.63 | ± | 1365.10 | 27,064.38 | ± | 108.21 | 25,348.00 | ± | 159.69 ** | 25,312.00 | ± | 588.39* | 25,248.75 | ± | 394.20 ** | 25,528.17 | ± | 142.82 ** |
| MIP-1β (pg/mL) | 2938.33 | ± | 207.54 | 22,371.33 | ± | 347.30 | 20,600.13 | ± | 502.24* | 21,125.63 | ± | 1433.70 | 20,115.33 | ± | 507.80 ** | 20,583.63 | ± | 380.70 ** |
| MIP-2 (pg/mL) | 62.50 | ± | 17.06 | 23,431.75 | ± | 383.83 | 21,235.17 | ± | 135.30 ** | 22,297.33 | ± | 706.70 ** | 21,889.83 | ± | 1104.75 * | 22,200.50 | ± | 680.97 * |
| RANTES (pg/mL) | 64.17 | ± | 11.45 | 11,415.50 | ± | 738.99 | 9406.67 | ± | 312.65* | 9832.33 | ± | 836.91 * | 9174.00 | ± | 829.30 | 10,096.00 | ± | 306.18 |
| VEGF (pg/mL) | 187.75 | ± | 32.95 | 3439.63 | ± | 322.20 | 3248.13 | ± | 302.35 | 3373.88 | ± | 186.46 | 3319.13 | ± | 570.31 | 3523.88 | ± | 506.10 |
| Chop mRNA (ratio) | 1.00 | ± | 0.42 | 27.09 | ± | 8.63 | 1.38 | ± | 0.12 ** | 0.77 | ± | 0.13 ** | 0.75 | ± | 0.32 ** | 1.75 | ± | 1.35 ** |
| Camk2α mRNA (ratio) | 1.01 | ± | 0.32 | 9.26 | ± | 2.72 | 2.91 | ± | 0.37 ** | 1.97 | ± | 0.11 ** | 2.32 | ± | 1.25 ** | 3.61 | ± | 1.51 * |
| Stat1 mRNA (ratio) | 1.00 | ± | 0.30 | 4.26 | ± | 0.06 | 0.76 | ± | 0.14 *** | 0.65 | ± | 0.09 *** | 0.63 | ± | 0.13 *** | 1.38 | ± | 0.23 *** |
| Stat3 mRNA (ratio) | 1.01 | ± | 0.31 | 3.36 | ± | 0.24 | 1.20 | ± | 0.41 *** | 1.20 | ± | 0.59 *** | 1.18 | ± | 0.46 *** | 1.16 | ± | 0.18 *** |
| Jak2 mRNA (ratio) | 1.00 | ± | 0.39 | 4.90 | ± | 0.92 | 0.72 | ± | 0.10 *** | 0.33 | ± | 0.01 *** | 0.32 | ± | 0.02 *** | 0.92 | ± | 0.10 *** |
| Fas mRNA (ratio) | 1.02 | ± | 0.18 | 51.52 | ± | 6.90 | 32.31 | ± | 6.04* | 14.63 | ± | 0.29 | 29.89 | ± | 6.34 ** | 21.44 | ± | 4.85 ** |
| c-Jun mRNA (ratio) | 1.02 | ± | 0.07 | 18.90 | ± | 1.71 | 1.96 | ± | 1.28 *** | 1.64 | ± | 0.81 *** | 1.39 | ± | 0.70 *** | 0.88 | ± | 0.51 *** |
| c-Fos mRNA (ratio) | 1.04 | ± | 0.13 | 48.73 | ± | 1.46 | 20.23 | ± | 3.05 *** | 10.51 | ± | 1.25 *** | 16.67 | ± | 0.29 *** | 27.88 | ± | 3.23 *** |
| Nos2 mRNA (ratio) | 1.00 | ± | 0.07 | 150.27 | ± | 4.90 | 75.71 | ± | 14.94 ** | 30.33 | ± | 7.73 *** | 84.76 | ± | 4.29 *** | 75.68 | ± | 3.51 *** |
| Ptgs2 mRNA (ratio) | 1.00 | ± | 0.03 | 1509.93 | ± | 34.75 | 515.01 | ± | 55.58 *** | 168.16 | ± | 13.40 *** | 520.00 | ± | 68.01 *** | 643.10 | ± | 53.53 *** |
| TLR3 mRNA (ratio) | 1.02 | ± | 0.11 | 5.30 | ± | 0.22 | 1.61 | ± | 0.05 *** | 1.29 | ± | 0.10 *** | 1.44 | ± | 0.20 *** | 3.43 | ± | 0.27 ** |
| TLR9 mRNA (ratio) | 1.01 | ± | 0.35 | 5.35 | ± | 0.44 | 0.86 | ± | 0.07 *** | 0.82 | ± | 0.04 *** | 0.99 | ± | 0.15 *** | 1.76 | ± | 0.26 *** |
| Treatment Group | Phosphorylation Level (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| STAT3 | p38 MAPK | IκB-α | |||||||
| LPS (1 µg/mL) only | 100.00 | ± | 0.61 | 100.00 | ± | 2.92 | 100.00 | ± | 2.93 |
| LPS + 25 µg/mL of CP | 66.09 | ± | 0.34 *** | 58.68 | ± | 1.10 *** | 43.99 | ± | 0.03 *** |
| LPS + 50 µg/mL of CP | 68.42 | ± | 1.72 *** | 56.75 | ± | 7.80 ** | 41.64 | ± | 3.12 *** |
| LPS + 100 µg/mL of CP | 63.03 | ± | 0.42 *** | 56.84 | ± | 2.17 *** | 41.34 | ± | 11.96 ** |
| LPS + Baicalein (25 µM) | 64.24 | ± | 1.03 | 51.66 | ± | 0.94 | 51.70 | ± | 2.22 |
| Gene Name | GenBank Accession Number |
|---|---|
| Chop | NM_007837 |
| Camk2α | NM_012920 |
| Stat1 | NM_009283.4 |
| Stat3 | NM_011486.5 |
| Jak2 | NM_001048177.3 |
| Fas | NM_007987 |
| c-Jun | NM_010591 |
| c-Fos | NM_010234 |
| Nos2 | NM_010927.3 |
| Ptgs2 | NM_011198 |
| TLR3 | NM_126166 |
| TLR9 | NM_031178.2 |
| β-Actin | NM_007393.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Kim, D.-H.; Park, W. Moutan Cortex Extract Modulates Macrophage Activation via Lipopolysaccharide-Induced Calcium Signaling and ER Stress-CHOP Pathway. Int. J. Mol. Sci. 2023, 24, 2062. https://doi.org/10.3390/ijms24032062
Kim H-J, Kim D-H, Park W. Moutan Cortex Extract Modulates Macrophage Activation via Lipopolysaccharide-Induced Calcium Signaling and ER Stress-CHOP Pathway. International Journal of Molecular Sciences. 2023; 24(3):2062. https://doi.org/10.3390/ijms24032062
Chicago/Turabian StyleKim, Hyun-Ju, Do-Hoon Kim, and Wansu Park. 2023. "Moutan Cortex Extract Modulates Macrophage Activation via Lipopolysaccharide-Induced Calcium Signaling and ER Stress-CHOP Pathway" International Journal of Molecular Sciences 24, no. 3: 2062. https://doi.org/10.3390/ijms24032062
APA StyleKim, H. -J., Kim, D. -H., & Park, W. (2023). Moutan Cortex Extract Modulates Macrophage Activation via Lipopolysaccharide-Induced Calcium Signaling and ER Stress-CHOP Pathway. International Journal of Molecular Sciences, 24(3), 2062. https://doi.org/10.3390/ijms24032062