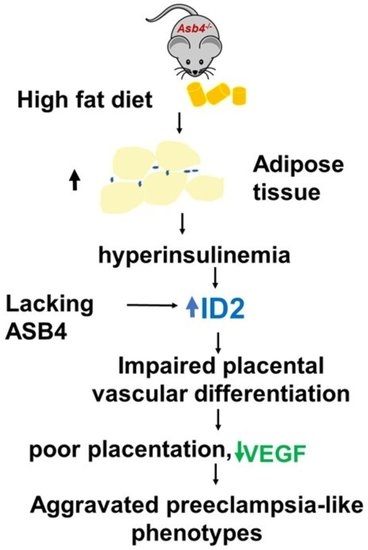
Insulin Elevates ID2 Expression in Trophoblasts and Aggravates Preeclampsia in Obese ASB4-Null Mice
Abstract
:1. Introduction
2. Results
2.1. Asb4−/− Female Mice Have More Fat Than WT Female Mice and a High-Fat Diet (HFD) Exacerbates It
2.2. A High-Fat Diet (HFD) Increases Circulating Levels of Cholesterol, Insulin, and Leptin in Asb4−/− Dams without Changes in Glucose Levels
2.3. HFD-Asb4−/− Dams Show Aggravated Preeclampsia-like Phenotypes
2.4. HFD-Asb4−/− Dams Have Decreased Vascular Endothelial Growth Factor (VEGF) Levels
2.5. Inhibitor of DNA-Binding Protein 2 (ID2) Amount in Placentas from HFD-Asb4−/− Dams Significantly Increase
2.6. Insulin, but Not Leptin, Increases ID2 in First-Trimester Trophoblasts
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Diet and Mating Strategy
4.3. Blood Pressure (BP) Measurements
4.4. Cell Culture
4.5. Other Procedures
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sibai, B.; Dekker, G.; Kupferminc, M. Pre-eclampsia. Lancet 2005, 365, 785–799. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.G.; Staff, A.C.; Roberts, J.M. Syncytiotrophoblast stress in preeclampsia: The convergence point for multiple pathways. Am. J. Obstet. Gynecol. 2022, 226, S907–S927. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V.; Keyes, K.M.; Wapner, R.J. Pre-eclampsia rates in the United States, 1980–2010: Age-period-cohort analysis. BMJ 2013, 347, f6564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodnar, L.M.; Ness, R.B.; Markovic, N.; Roberts, J.M. The risk of preeclampsia rises with increasing prepregnancy body mass index. Ann. Epidemiol. 2005, 15, 475–482. [Google Scholar] [CrossRef]
- Ayala-Ramirez, P.; Serrano, N.; Barrera, V.; Bejarano, J.P.; Silva, J.L.; Martinez, R.; Gil, F.; Olaya, C.M.; Garcia-Robles, R. Risk factors and fetal outcomes for preeclampsia in a Colombian cohort. Heliyon 2020, 6, e05079. [Google Scholar] [CrossRef]
- Yang, Y.; Le Ray, I.; Zhu, J.; Zhang, J.; Hua, J.; Reilly, M. Preeclampsia Prevalence, Risk Factors, and Pregnancy Outcomes in Sweden and China. JAMA Netw. Open 2021, 4, e218401. [Google Scholar] [CrossRef]
- Roberts, J.M.; Bodnar, L.M.; Patrick, T.E.; Powers, R.W. The Role of Obesity in Preeclampsia. Pregnancy Hypertens. 2011, 1, 6–16. [Google Scholar] [CrossRef] [Green Version]
- Townley-Tilson, W.H.; Wu, Y.; Ferguson, J.E., 3rd; Patterson, C. The ubiquitin ligase ASB4 promotes trophoblast differentiation through the degradation of ID2. PLoS ONE 2014, 9, e89451. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, J.E., 3rd; Wu, Y.; Smith, K.; Charles, P.; Powers, K.; Wang, H.; Patterson, C. ASB4 is a hydroxylation substrate of FIH and promotes vascular differentiation via an oxygen-dependent mechanism. Mol. Cell. Biol. 2007, 27, 6407–6419. [Google Scholar] [CrossRef] [Green Version]
- Ling, F.; Kang, B.; Sun, X.H. Id proteins: Small molecules, mighty regulators. Curr. Top. Dev. Biol. 2014, 110, 189–216. [Google Scholar]
- Liu, Y.P.; Burleigh, D.; Durning, M.; Hudson, L.; Chiu, I.M.; Golos, T.G. Id2 is a primary partner for the E2-2 basic helix-loop-helix transcription factor in the human placenta. Mol. Cell. Endocrinol. 2004, 222, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Fushima, T.; Oyanagi, G.; Townley-Tilson, H.W.; Sato, E.; Nakada, H.; Oe, Y.; Hagaman, J.R.; Wilder, J.; Li, M.; et al. Nicotinamide benefits both mothers and pups in two contrasting mouse models of preeclampsia. Proc. Natl. Acad. Sci. USA 2016, 113, 13450–13455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faulkner, J.L.; Wright, D.; Antonova, G.; Jaffe, I.Z.; Kennard, S.; Belin de Chantemele, E.J. Midgestation Leptin Infusion Induces Characteristics of Clinical Preeclampsia in Mice, Which Is Ablated by Endothelial Mineralocorticoid Receptor Deletion. Hypertension 2022, 79, 1536–1547. [Google Scholar] [CrossRef] [PubMed]
- Alston, M.C.; Redman, L.M.; Sones, J.L. An Overview of Obesity, Cholesterol, and Systemic Inflammation in Preeclampsia. Nutrients 2022, 14, 2087. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.M.; Carter, N.D.; Wilson, C.A.; Scaramuzzi, R.J.; Stock, M.J.; Murray, J.F. Leptin expression in the fetus and placenta during mouse pregnancy. Placenta 2005, 26, 47–52. [Google Scholar] [CrossRef]
- Matsuda, Y.; Ogawa, M.; Nakai, A.; Hayashi, M.; Satoh, S.; Matsubara, S. Fetal/Placental weight ratio in term Japanese pregnancy: Its difference among gender, parity, and infant growth. Int. J. Med. Sci. 2015, 12, 301–305. [Google Scholar] [CrossRef] [Green Version]
- Sui, X.; Zhang, L.; Zhang, X.F.; Zhang, Y. TRIB3-Regulated Akt Signal Pathway Affects Trophoblast Invasion in the Development of Preeclampsia. Am. J. Perinatol. 2021. [Google Scholar] [CrossRef]
- Li, F.; Kakoki, M.; Smid, M.; Boggess, K.; Wilder, J.; Hiller, S.; Bounajim, C.; Parnell, S.E.; Sulik, K.K.; Smithies, O.; et al. Causative Effects of Genetically Determined High Maternal/Fetal Endothelin-1 on Preeclampsia-Like Conditions in Mice. Hypertension 2018, 71, 894–903. [Google Scholar] [CrossRef]
- Woods, A.K.; Hoffmann, D.S.; Weydert, C.J.; Butler, S.D.; Zhou, Y.; Sharma, R.V.; Davisson, R.L. Adenoviral delivery of VEGF121 early in pregnancy prevents spontaneous development of preeclampsia in BPH/5 mice. Hypertension 2011, 57, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Huda, S.S.; Jordan, F.; Bray, J.; Love, G.; Payne, R.; Sattar, N.; Freeman, D.J. Visceral adipose tissue activated macrophage content and inflammatory adipokine secretion is higher in pre-eclampsia than in healthy pregnancys. Clin. Sci. 2017, 131, 1529–1540. [Google Scholar] [CrossRef] [Green Version]
- Park, K.W.; Waki, H.; Villanueva, C.J.; Monticelli, L.A.; Hong, C.; Kang, S.; MacDougald, O.A.; Goldrath, A.W.; Tontonoz, P. Inhibitor of DNA binding 2 is a small molecule-inducible modulator of peroxisome proliferator-activated receptor-gamma expression and adipocyte differentiation. Mol. Endocrinol. 2008, 22, 2038–2048. [Google Scholar] [CrossRef]
- Mathew, D.; Zhou, P.; Pywell, C.M.; van der Veen, D.R.; Shao, J.; Xi, Y.; Bonar, N.A.; Hummel, A.D.; Chapman, S.; Leevy, W.M.; et al. Ablation of the ID2 gene results in altered circadian feeding behavior, and sex-specific enhancement of insulin sensitivity and elevated glucose uptake in skeletal muscle and brown adipose tissue. PLoS ONE 2013, 8, e73064. [Google Scholar] [CrossRef] [Green Version]
- Palei, A.C.; Spradley, F.T.; Granger, J.P. Euglycemic hyperinsulinemia increases blood pressure in pregnant rats independent of placental antiangiogenic and inflammatory factors. Am. J. Hypertens. 2013, 26, 1445–1451. [Google Scholar] [CrossRef] [Green Version]
- Takao, T.; Asanoma, K.; Kato, K.; Fukushima, K.; Tsunematsu, R.; Hirakawa, T.; Matsumura, S.; Seki, H.; Takeda, S.; Wake, N. Isolation and characterization of human trophoblast side-population (SP) cells in primary villous cytotrophoblasts and HTR-8/SVneo cell line. PLoS ONE 2011, 6, e21990. [Google Scholar] [CrossRef] [Green Version]
- Vega, M.; Mauro, M.; Williams, Z. Direct toxicity of insulin on the human placenta and protection by metformin. Fertil. Steril. 2019, 111, 489–496.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Lu, S.; Yang, C.; Li, N.; Chen, X.; He, J.; Liu, X.; Ding, Y.; Tong, C.; Peng, C.; et al. Hyperinsulinemia restrains endometrial angiogenesis during decidualization in early pregnancy. J. Endocrinol. 2019, 243, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Hale, L.J.; Hurcombe, J.; Lay, A.; Santamaria, B.; Valverde, A.M.; Saleem, M.A.; Mathieson, P.W.; Welsh, G.I.; Coward, R.J. Insulin directly stimulates VEGF-A production in the glomerular podocyte. Am. J. Physiol. Renal. Physiol. 2013, 305, F182–F188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weedon-Fekjaer, M.S.; Sheng, Y.; Sugulle, M.; Johnsen, G.M.; Herse, F.; Redman, C.W.; Lyle, R.; Dechend, R.; Staff, A.C. Placental miR-1301 is dysregulated in early-onset preeclampsia and inversely correlated with maternal circulating leptin. Placenta 2014, 35, 709–717. [Google Scholar] [CrossRef]
- Masuyama, H.; Hiramatsu, Y. Treatment with a constitutive androstane receptor ligand ameliorates the signs of preeclampsia in high-fat diet-induced obese pregnant mice. Mol. Cell. Endocrinol. 2012, 348, 120–127. [Google Scholar] [CrossRef]
- Palei, A.C.; Spradley, F.T.; Granger, J.P. Role of Nitric Oxide Synthase on Blood Pressure Regulation and Vascular Function in Pregnant Rats on a High-Fat Diet. Am. J. Hypertens. 2017, 30, 240–248. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.N.; Yang, Z.; Ma, R.Q. Effect of high-fat diet on liver and placenta fatty infiltration in early onset preeclampsia-like mouse model. Chin. Med. J. 2012, 125, 3532–3538. [Google Scholar] [PubMed]
- Janatpour, M.J.; McMaster, M.T.; Genbacev, O.; Zhou, Y.; Dong, J.; Cross, J.C.; Israel, M.A.; Fisher, S.J. Id-2 regulates critical aspects of human cytotrophoblast differentiation, invasion and migration. Development 2000, 127, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Selesniemi, K.; Albers, R.E.; Brown, T.L. Id2 Mediates Differentiation of Labyrinthine Placental Progenitor Cell Line, SM10. Stem Cells Dev. 2016, 25, 959–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belfiore, F.; Iannello, S.; Volpicelli, G. Insulin sensitivity indices calculated from basal and OGTT-induced insulin, glucose, and FFA levels. Mol. Genet. Metab. 1998, 63, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Jeyarajah, M.J.; Jaju Bhattad, G.; Hillier, D.M.; Renaud, S.J. The Transcription Factor OVOL2 Represses ID2 and Drives Differentiation of Trophoblast Stem Cells and Placental Development in Mice. Cells 2020, 9, 840. [Google Scholar] [CrossRef] [Green Version]
- Proud, C.G.; Denton, R.M. Molecular mechanisms for the control of translation by insulin. Biochem. J. 1997, 328 Pt 2, 329–341. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.; Nunes, C.; Correia-Branco, A.; Araujo, J.R.; Martel, F. Insulin Exhibits an Antiproliferative and Hypertrophic Effect in First Trimester Human Extravillous Trophoblasts. Reprod. Sci. 2017, 24, 582–594. [Google Scholar] [CrossRef]
- Nandi, P.; Lim, H.; Torres-Garcia, E.J.; Lala, P.K. Human trophoblast stem cell self-renewal and differentiation: Role of decorin. Sci. Rep. 2018, 8, 8977. [Google Scholar] [CrossRef] [Green Version]
- Li, J.Y.; Chai, B.; Zhang, W.; Wu, X.; Zhang, C.; Fritze, D.; Xia, Z.; Patterson, C.; Mulholland, M.W. Ankyrin repeat and SOCS box containing protein 4 (Asb-4) colocalizes with insulin receptor substrate 4 (IRS4) in the hypothalamic neurons and mediates IRS4 degradation. BMC Neurosci. 2011, 12, 95. [Google Scholar] [CrossRef] [Green Version]
- Li, J.Y.; Chai, B.X.; Zhang, W.; Wang, H.; Mulholland, M.W. Expression of ankyrin repeat and suppressor of cytokine signaling box protein 4 (Asb-4) in proopiomelanocortin neurons of the arcuate nucleus of mice produces a hyperphagic, lean phenotype. Endocrinology 2010, 151, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Vagena, E.; Crneta, J.; Engstrom, P.; He, L.; Yulyaningsih, E.; Korpel, N.L.; Cheang, R.T.; Bachor, T.P.; Huang, A.; Michel, G.; et al. ASB4 modulates central melanocortinergic neurons and calcitonin signaling to control satiety and glucose homeostasis. Sci. Signal 2022, 15, eabj8204. [Google Scholar] [CrossRef] [PubMed]
- Kriebs, A. Obesity-associated ASB4 controls satiety. Nat. Rev. Endocrinol. 2022, 18, 456. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Bahnson, E.M.; Wilder, J.; Siletzky, R.; Hagaman, J.; Nickekeit, V.; Hiller, S.; Ayesha, A.; Feng, L.; Levine, J.S.; et al. Oral high dose vitamin B12 decreases renal superoxide and post-ischemia/reperfusion injury in mice. Redox Biol. 2020, 32, 101504. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, C.H.; Wang, J.G.; Thai, T.; Boysen, G.; Xu, L.; Turner, A.L.; Wolberg, A.S.; Mackman, N.; Maeda, N.; et al. Elevated tissue factor expression contributes to exacerbated diabetic nephropathy in mice lacking eNOS fed a high fat diet. J. Thromb. Haemost. 2010, 8, 2122–2132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, C.H.; Hawley, T.S.; Hawley, R.G.; MacDougall, J.R.; Kerbel, R.S.; Khoo, N.; Lala, P.K. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp. Cell Res. 1993, 206, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.S.; Lee, G.S.; Nam, S.Y.; Kim, S.J. Progesterone Inhibits Leptin-Induced Invasiveness of BeWo Cells. Int. J. Med. Sci. 2015, 12, 773–779. [Google Scholar] [CrossRef] [Green Version]
- Toro, A.R.; Maymo, J.L.; Ibarbalz, F.M.; Perez-Perez, A.; Maskin, B.; Faletti, A.G.; Sanchez-Margalet, V.; Varone, C.L. Leptin is an anti-apoptotic effector in placental cells involving p53 downregulation. PLoS ONE 2014, 9, e99187. [Google Scholar] [CrossRef]
- Takahashi, N.; Li, F.; Hua, K.; Deng, J.; Wang, C.H.; Bowers, R.R.; Bartness, T.J.; Kim, H.S.; Harp, J.B. Increased energy expenditure, dietary fat wasting, and resistance to diet-induced obesity in mice lacking renin. Cell Metab. 2007, 6, 506–512. [Google Scholar] [CrossRef] [Green Version]
- Waldo, K.L.; Kumiski, D.; Kirby, M.L. Cardiac neural crest is essential for the persistence rather than the formation of an arch artery. Dev. Dyn. 1996, 205, 281–292. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayashima, Y.; Townley-Tilson, W.H.D.; Vora, N.L.; Boggess, K.; Homeister, J.W.; Maeda-Smithies, N.; Li, F. Insulin Elevates ID2 Expression in Trophoblasts and Aggravates Preeclampsia in Obese ASB4-Null Mice. Int. J. Mol. Sci. 2023, 24, 2149. https://doi.org/10.3390/ijms24032149
Kayashima Y, Townley-Tilson WHD, Vora NL, Boggess K, Homeister JW, Maeda-Smithies N, Li F. Insulin Elevates ID2 Expression in Trophoblasts and Aggravates Preeclampsia in Obese ASB4-Null Mice. International Journal of Molecular Sciences. 2023; 24(3):2149. https://doi.org/10.3390/ijms24032149
Chicago/Turabian StyleKayashima, Yukako, W. H. Davin Townley-Tilson, Neeta L. Vora, Kim Boggess, Jonathon W. Homeister, Nobuyo Maeda-Smithies, and Feng Li. 2023. "Insulin Elevates ID2 Expression in Trophoblasts and Aggravates Preeclampsia in Obese ASB4-Null Mice" International Journal of Molecular Sciences 24, no. 3: 2149. https://doi.org/10.3390/ijms24032149
APA StyleKayashima, Y., Townley-Tilson, W. H. D., Vora, N. L., Boggess, K., Homeister, J. W., Maeda-Smithies, N., & Li, F. (2023). Insulin Elevates ID2 Expression in Trophoblasts and Aggravates Preeclampsia in Obese ASB4-Null Mice. International Journal of Molecular Sciences, 24(3), 2149. https://doi.org/10.3390/ijms24032149