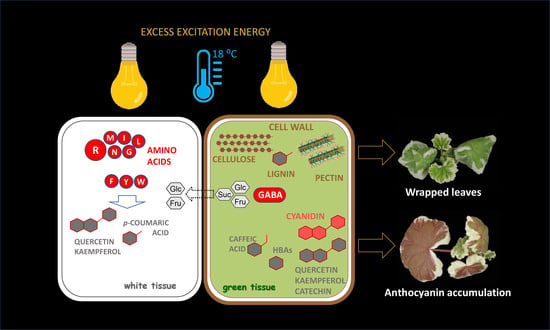
Carbon and Nitrogen Allocation between the Sink and Source Leaf Tissue in Response to the Excess Excitation Energy Conditions
Abstract
:1. Introduction
2. Results
2.1. Effect of EEE on the Chlorophyll Fluorescence Parameters
2.2. Effect of EEE on Epidermal Flavonoid and Chlorophyll Contents in P. zonale Leaves
2.3. Effect of EEE Conditions on Phenolic Content
2.4. Morphological Changes in P. zonale Leaves Induced by EEE Conditions
2.5. Effects of EEE Conditions on the Cell Wall Components of P. zonale Leaves
2.6. Effect of EEE Conditions on the Free Amino Acid Content
2.7. Gene Expression
3. Discussion
3.1. Chlorophyll Content and Chlorophyll Fluorescence Parameters
3.2. Phenolics
3.3. Cell Wall Constituents
3.4. Amino Acids
4. Materials and Methods
4.1. Plant Material and Treatment
4.2. Morphological Leaf and Chlorophyll Fluorescence Parameters
4.3. Dynamics of Epidermal Flavonoids and Total Chlorophyll Accumulation
4.4. Analysis of the Polyphenols
4.5. Cell Wall Isolation and Purification
4.6. Analysis of Cell-Wall-Bound Phenolics
4.7. Infrared (IR) Spectroscopy of the Cell Wall Samples
4.8. Amino Acid Analysis and the Enrichment of Amino Acids with 15N
4.9. qPCR
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Popova, A.V.; Stefanov, M.; Ivanov, A.G.; Velitchkova, M. The role of alternative electron pathways for effectiveness of photosynthetic performance of Arabidopsis thaliana, wt and Lut2, under low temperature and high light intensity. Plants 2022, 11, 2318. [Google Scholar] [CrossRef] [PubMed]
- Driever, S.M.; Baker, N.R. The water–water cycle in leaves is not a major alternative electron sink for dissipation of excess excitation energy when CO2 assimilation is restricted. Plant Cell Environ. 2011, 34, 837–846. [Google Scholar] [CrossRef] [PubMed]
- García-Plazaola, J.I.; Esteban, R.; Fernández-Marín, B.; Kranner, I.; Porcar-Castell, A. Thermal energy dissipation and xanthophyll cycles beyond the Arabidopsis model. Photosynth. Res. 2012, 113, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Roach, T.; Krieger-Liszkay, A. Regulation of photosynthetic electron transport and photoinhibition. Curr. Protein Pept. Sci. 2014, 15, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Exposito-Rodriguez, M.; Laissue, P.P.; Yvon-Durocher, G.; Smirnoff, N.; Mullineaux, P.M. Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nat. Commun. 2017, 8, 49. [Google Scholar] [CrossRef] [Green Version]
- Vojta, L.; Carić, D.; Cesar, V.; Dunić, J.A.; Lepeduš, H.; Kveder, M.; Fulgosi, H. TROL-FNR interaction reveals alternative pathways of electron partitioning in photosynthesis. Sci. Rep. 2015, 5, 10085. [Google Scholar] [CrossRef] [Green Version]
- Martins, S.C.; Araújo, W.L.; Tohge, T.; Fernie, A.R.; DaMatta, F.M. In high-light-acclimated coffee plants the metabolic machinery is adjusted to avoid oxidative stress rather than to benefit from extra light enhancement in photosynthetic yield. PLoS ONE 2014, 9, e94862. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.L.; Hu, Y.Y.; Luo, H.H.; Chow, W.S.; Zhang, W.F. Two distinct strategies of cotton and soybean differing leaf movement to perform photosynthesis under water stress. Funct. Plant Biol. 2011, 38, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Demmig-Adams, B.; Stewart, J.J.; López-Pozo, M.; Polutchko, S.K.; Adams III, W.W. Zeaxanthin, a molecule for photoprotection in many different environments. Molecules 2020, 25, 5825. [Google Scholar] [CrossRef]
- Hernández, I.; Van Breusegem, F. Opinion on the possible role of flavonoids as energy escape valves: Novel tools for nature’s Swiss army knife? Plant Sci. 2010, 179, 297–301. [Google Scholar] [CrossRef]
- Hernández, I.; Alegre, L.; Van Breusegem, F.; Munné-Bosch, S. How relevant are flavonoids as antioxidants in plants? Trends Plant Sci. 2009, 141, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.O.; Gould, K.S. Anthocyanins in leaves: Light attenuators or antioxidants? Funct. Plant Biol. 2003, 30, 865–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef] [Green Version]
- Agati, G.; Matteini, P.; Goti, A.; Tattini, M. Chloroplast-located flavonoids can scavenge singlet oxygen. New Phytol. 2007, 174, 77–89. [Google Scholar] [CrossRef]
- Ferreres, F.; Figueiredo, R.; Bettencourt, S.; Carqueijeiro, I.; Oliveira, J.; Gil-Izquierdo, A.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Duarte, P.; et al. Identification of phenolic compounds in isolated vacuoles of the medicinal plant Catharanthus roseus and their interaction with vacuolar class III peroxidase: An H2O2 affair? J. Exp. Bot. 2011, 62, 2841–2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veljović Jovanović, S.; Kukavica, B.; Vidović, M.; Morina, F.; Menckhoff, L. Class III peroxidases: Functions, localization and redox regulation of isoenzymes. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer: Cham, Switzerland; New York City, NY, USA, 2018; pp. 269–300. [Google Scholar]
- Guidi, L.; Brunetti, C.; Fini, A.; Agati, G.; Ferrini, F.; Gori, A.; Tattini, M. UV radiation promotes flavonoid biosynthesis, while negatively affecting the biosynthesis and the de-epoxidation of xanthophylls: Consequence for photoprotection? Environ. Exp. Bot. 2016, 127, 14–25. [Google Scholar] [CrossRef]
- Falcioni, R.; Moriwaki, T.; de Oliveira, D.M.; Andreotti, G.C.; de Souza, L.A.; Dos Santos, W.D.; Bonato, C.M.; Antunes, W.C. Increased gibberellins and light levels promotes cell wall thickness and enhance lignin deposition in xylem fibers. Front. Plant Sci. 2018, 9, 1391. [Google Scholar] [CrossRef] [Green Version]
- Milić, D.; Pantelić, A.; Banović-Djeri, B.; Samardžić, J.; Vidović, M. Contrasting metabolisms in green and white leaf sec-tors of variegated Pelargonium zonale—an integrated transcriptomic and metabolomic study. Int. J. Mol. Sci. 2022, 23. Under review. [Google Scholar]
- Vidović, M.; Morina, F.; Milić, S.; Albert, A.; Zechmann, B.; Tosti, T.; Winkler, J.B.; Veljović Jovanović, S. Carbon allocation from source to sink leaf tissue in relation to flavonoid biosynthesis in variegated Pelargonium zonale under UV-B radiation and high PAR intensity. Plant Physiol. Biochem. 2015, 93, 44–55. [Google Scholar] [CrossRef]
- Vidović, M.; Morina, F.; Milić, S.; Vuleta, A.; Zechmann, B.; Prokić, L.; Veljović Jovanović, S. Characterisation of antioxidants in photosynthetic and non-photosynthetic leaf tissues of variegated Pelargonium zonale plants. Plant Biol. (Stuttg) 2016, 18, 669–680. [Google Scholar] [CrossRef]
- Vidović, M.; Morina, F.; Prokić, L.; Milić-Komić, S.; Živanović, B.; Veljović Jovanović, S. Antioxidative response in variegated Pelargonium zonale leaves and generation of extracellular H2O2 in (peri) vascular tissue induced by sunlight and paraquat. J. Plant Physiol. 2016, 206, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Tcherkez, G.; Guérard, F.; Gilard, F.; Lamothe, M.; Mauve, C.; Gout, E.; Bligny, R. Metabolomic characterization of the functional division of nitrogen metabolism in variegated leaves. Funct. Plant Biol. 2012, 39, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Vidović, M.; Battisti, I.; Pantelić, A.; Morina, F.; Arrigoni, G.; Masi, A.; Veljović Jovanović, S. Desiccation tolerance in Ramonda serbica Panc.: An integrative transcriptomic, proteomic, metabolite and photosynthetic study. Plants 2022, 11, 1199. [Google Scholar] [CrossRef] [PubMed]
- Toldi, D.; Gyugos, M.; Darkó, É.; Szalai, G.; Gulyás, Z.; Gierczik, K.; Székely, A.; Boldizsár, Á.; Galiba, G.; Müller, M.; et al. Light intensity and spectrum affect metabolism of glutathione and amino acids at transcriptional level. PLoS ONE 2019, 14, e0227271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagata, N.; Tanaka, R.; Tanaka, A. The major route for chlorophyll synthesis includes [3,8-divinyl]-chlorophyllide a reduction in Arabidopsis thaliana. Plant Cell Physiol. 2007, 48, 1803–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Han, Q.; Ding, C.; Huang, Y.; Liao, J.; Chen, T.; Feng, S.; Zhou, L.; Zhang, Y.; Chen, Y.; et al. Effect of low temperature on chlorophyll biosynthesis and chloroplast biogenesis of rice seedlings during greening. Int. J. Mol. Sci. 2020, 21, 1390. [Google Scholar] [CrossRef] [Green Version]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu Rev. Plant. Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Asada, K. The water–water cycle as alternative photon and electron sinks. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1419–1431. [Google Scholar] [CrossRef] [Green Version]
- Veljović-Jovanović, S.; Vidović, M.; Morina, F. Ascorbate as a key player in plant abiotic stress response and tolerance. In Ascorbic Acid in Plant Growth, Development and Stress Tolerance; Hossain, M., Munné-Bosch, S., Burritt, D., Diaz-Vivancos, P., Fujita, M., Lorence, A., Eds.; Springer: Cham, Switzerland; New York City, NY, USA, 2017; pp. 47–109. [Google Scholar]
- Zhang, H.; Feng, P.; Yang, W.; Sui, X.; Li, X.; Li, W.; Zhang, R.; Gu, S.; Xu, N. Effects of flooding stress on the photosynthetic apparatus of leaves of two Physocarpus cultivars. J. For. Res. 2018, 29, 1049–1059. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Temperature dependence of violaxanthin de-epoxidation and non-photochemical fluorescence quenching in intact leaves of Gossypium hirsutum L. and Malva parviflora L. Planta 1991, 184, 226–234. [Google Scholar] [CrossRef]
- Kanazawa, A.; Kramer, D.M. In vivo modulation of nonphotochemical exciton quenching (NPQ) by regulation of the chloroplast ATP synthase. Proc. Natl. Acad. Sci. USA 2002, 99, 12789–12794. [Google Scholar] [CrossRef] [PubMed]
- Kohzuma, K.; Dal Bosco, C.; Meurer, J.; Kramer, D.M. Light-and metabolism-related regulation of the chloroplast ATP synthase has distinct mechanisms and functions. J. Biol. Chem. 2013, 288, 13156–13163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.; Sultana, N.; Paszkiewicz, K.; Florance, H.; Smirnoff, N. The influence of ascorbate on anthocyanin accumulation during high light acclimation in Arabidopsis thaliana: Further evidence for redox control of anthocyanin synthesis. Plant Cell Environ. 2012, 35, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Stefano, G.; Biricolti, S.; Tattini, M. Mesophyll distribution of ‘antioxidant’flavonoid glycosides in Ligustrum vulgare leaves under contrasting sunlight irradiance. Ann. Bot. 2009, 104, 853–861. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, L.B.d.S.; Tattini, M. Beyond photoprotection: The multifarious roles of flavonoids in plant terrestrialization. Int. J. Mol. Sci. 2022, 23, 5284. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Takahama, U. Oxidation of vacuolar and apoplastic phenolic substrates by peroxidase: Physiological significance of the oxidation reactions. Phytochem Rev. 2004, 3, 207–219. [Google Scholar] [CrossRef]
- Dixon, R.A.; Xie, D.Y.; Sharma, S.B. Proanthocyanidins–a final frontier in flavonoid research? New Phytologist. 2005, 165, 9–28. [Google Scholar] [CrossRef] [Green Version]
- Landi, M.; Agati, G.; Fini, A.; Guidi, L.; Sebastiani, F.; Tattini, M. Unveiling the shade nature of cyanic leaves: A view from the “blue absorbing side” of anthocyanins. Plant, Cell Environ. 2021, 44, 1119–1129. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Assembly and enlargement of the primary cell wall in plants. Annu. Rev. Cell Dev. Biol. 1997, 13, 171–201. [Google Scholar] [CrossRef] [Green Version]
- Cerović, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Vidović, M.; Morina, F.; Veljović-Jovanović, S. Stimulation of various phenolics in plants under ambient UV-B radiation. In UV-B Radiation: From Environmental Stressor to Regulator of Plant Growth; Singh, V.P., Singh, S., Prasad, S.M., Parihar, P., Eds.; Wiley-Blackwell: Chichester, UK, 2017; pp. 9–56. [Google Scholar]
- Zhang, G.; Hou, X.; Wang, L.; Xu, J.; Chen, J.; Fu, X.; Shen, N.; Nian, J.; Jiang, Z.; Hu, J.; et al. PHOTO-SENSITIVE LEAF ROLLING 1 encodes a polygalacturonase that modifies cell wall structure and drought tolerance in rice. New Phytologist. 2021, 229, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Onoda, Y.; Wright, I.J.; Evans, J.R.; Hikosaka, K.; Kitajima, K.; Niinemets, Ü.; Poorter, H.; Tosens, T.; Niinemets, Ü. Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol. 2017, 214, 1447–1463. [Google Scholar] [CrossRef] [Green Version]
- Schopfer, P.; Lapierre, C.; Nolte, T. Light-controlled growth of the maize seedling mesocotyl: Mechanical cell-wall changes in the elongation zone and related changes in lignification. Physiol. Plant. 2001, 111, 83–92. [Google Scholar] [CrossRef]
- Noctor, G. Manipulation of glutathione and amino acid biosynthesis in the chloroplast. Plant Physiol. 1998, 118, 471–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jänkänpää, H.J.; Mishra, Y.; Schroder, W.P.; Jansson, S. Metabolic profiling reveals metabolic shifts in Arabidopsis plants grown under different light conditions. Plant Cell Environ. 2012, 35, 1824–1836. [Google Scholar] [CrossRef]
- Suzuki, A.; Rioual, S.; Lemarchand, S.; Godfroy, N.; Roux, Y.; Boutin, J.P.; Rothstein, S. Regulation by light and metabolites of ferredoxin-dependent glutamate synthase in maize. Physiol Plant. 2001, 112, 524–530. [Google Scholar] [CrossRef]
- Dhakal, R.; Baek, K.H. Metabolic alternation in the accumulation of free amino acids and γ-aminobutyric acid in postharvest mature green tomatoes following irradiation with blue light. Hortic. Environ. Biotechnol. 2014, 55, 36–41. [Google Scholar] [CrossRef]
- Abadie, C.; Lamothe, M.; Mauve, C.; Gilard, F.; Tcherkez, G. Leaf green-white variegation is advantageous under N deprivation in Pelargonium hortorum. Funct. Plant Biol. 2015, 42, 543–551. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nesi, A.N.; Araújo, W.L.; Braun, H.P. Amino acid catabolism in plants. Mol Plant. 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.; Todd, C.D.; Trovato, M.; Forlani, G.; Funck, D. Physiological implications of arginine metabolism in plants. Front. Plant Sci. 2015, 6, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramesh, S.A.; Tyerman, S.D.; Gilliham, M.; Xu, B. γ-Aminobutyric acid (GABA) signalling in plants. Cell. Mol. Life Sci. 2017, 74, 1577–1603. [Google Scholar] [CrossRef]
- Novitskaya, L.; Trevanion, S.; Driscoll, S.; Foyer, C.; Noctor, G. How does photorespiration modulate leaf amino acid contents? A dual approach through modelling and metabolite analysis. Plant Cell Environ. 2002, 25, 821–835. [Google Scholar] [CrossRef] [Green Version]
- Ros, R.; Muñoz-Bertomeu, J.; Krueger, S. Serine in plants: Biosynthesis, metabolism, and functions. Trends Plant Sci. 2014, 19, 564–569. [Google Scholar] [CrossRef]
- Lea, P.J.; Sodek, L.; Parry, M.A.; Shewry, P.R.; Halford, N.G. Asparagine in plants. Ann. Appl. Biol. 2007, 150, 1–26. [Google Scholar] [CrossRef]
- Araújo, W.L.; Ishizaki, K.; Nunes-Nesi, A.; Larson, T.R.; Tohge, T.; Krahnert, I.; Witt, S.; Obata, T.; Schauer, N.; Graham, I.A.; et al. Identification of the 2-hydroxyglutarate and isovaleryl-CoA dehydrogenases as alternative electron donors linking lysine catabolism to the electron transport chain of Arabidopsis mitochondria. Plant Cell 2010, 22, 1549–1563. [Google Scholar] [CrossRef] [Green Version]
- Cao, P.; Kim, S.J.; Xing, A.; Schenck, C.A.; Liu, L.; Jiang, N.; Wang, J.; Last, R.L.; Brandizzi, F. Homeostasis of branched-chain amino acids is critical for the activity of TOR signaling in Arabidopsis. eLife 2019, 8, e50747. [Google Scholar] [CrossRef]
- Chen, G.H.; Liu, M.J.; Xiong, Y.; Sheen, J.; Wu, S.H. TOR and RPS6 transmit light signals to enhance protein translation in deetiolating Arabidopsis seedlings. Proc. Natl. Acad. Sci. USA 2018, 115, 12823–12828. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, A.; Janocha, D.; Dong, Y.; Medzihradszky, A.; Schöne, S.; Daum, G.; Suzaki, T.; Forner, J.; Langenecker, T.; Rempel, E.; et al. Integration of light and metabolic signals for stem cell activation at the shoot apical meristem. eLife 2016, 5, e17023. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Coruzzi, G.; Last, R. Amino acids. In Biochemistry and Molecular Biology of Plants; Buchanan, B.B., Gruissem, W., Jones, R., Eds.; American Society of Plant Physiology Press: Rockville, MD, USA, 2000; pp. 358–410. [Google Scholar]
- Sade, N.; Galkin, E.; Moshelion, M. Measuring Arabidopsis, tomato and barley leaf relative water content (RWC). Bio-Protocol 2015, 5, e1451. [Google Scholar] [CrossRef] [Green Version]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Ann. Rev. Plant Biol. 2008, 59, 89. [Google Scholar] [CrossRef] [Green Version]
- Bogdanovic, J.; Dikanovic, D.; Maksimovic, V.; Tufegdzic, S.; Dokovic, D.; Isajev, V.; Radotic, K. Phenolics, lignin content and peroxidase activity in Picea omorika lines. Biol. Plant. 2006, 50, 461–464. [Google Scholar] [CrossRef]
- Alonso-Simón, A.; García-Angulo, P.; Mélida, H.; Encina, A.; Álvarez, J.M.; Acebes, J.L. The use of FTIR spectroscopy to monitor modifications in plant cell wall architecture caused by cellulose biosynthesis inhibitors. Plant Signal. Behav. 2011, 6, 1104–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobille, H.; Fustec, J.; Robins, R.J.; Cukier, C.; Limami, A.M. Effect of water availability on changes in root amino acids and associated rhizosphere on root exudation of amino acids in Pisum sativum L. Phytochemistry 2019, 161, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Cukier, C.; Lea, P.J.; Canas, R.; Marmagne, A.; Limami, A.M.; Hirel, B. Labeling maize (Zea mays L.) leaves with 15NH4+ and monitoring nitrogen incorporation into amino acids by GC/MS analysis. Curr. Protoc. Plant Biol. 2018, 3, e20073. [Google Scholar] [CrossRef] [PubMed]
- Vidović, M.; Ćuković, K. Isolation of high-quality RNA from recalcitrant leaves of variegated and resurrection plants. 3 Biotech. 2020, 10, 286–294. [Google Scholar] [CrossRef]

















| G_5 | GLL_9 | GLL_13 | GHL_9 | GHL_13 | W_5 | WLL_9 | WLL_13 | WHL_9 | WHL_13 | |
|---|---|---|---|---|---|---|---|---|---|---|
| HL | 3.6 ± 0.3 a | 4.8 ± 0.5 ab | 5.4 ± 0.2 abc | 6.5 ± 0.2 bcde | 7.4 ± 0.4 cdef | 8.6 ± 0.6 ef | 6.2 ± 0.3 bcd | 9.6 ± 0.4 fg | 7.8 ± 0.9 def | 11.4 ± 0.9 g |
| Cold + HL | 5.1 ± 0.4 a | 6.9 ± 0.3 a | 7.3 ± 0.7 a | 5.8 ± 0.7 a | 7.3 ± 0.7 a | 17.3 ± 1.8 b | 15.6 ± 0.6 b | 16.1 ± 1.1 b | 13.1 ± 0.7 b | 22.4 ± 2.1 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milić, D.; Živanović, B.; Samardžić, J.; Nikolić, N.; Cukier, C.; Limami, A.M.; Vidović, M. Carbon and Nitrogen Allocation between the Sink and Source Leaf Tissue in Response to the Excess Excitation Energy Conditions. Int. J. Mol. Sci. 2023, 24, 2269. https://doi.org/10.3390/ijms24032269
Milić D, Živanović B, Samardžić J, Nikolić N, Cukier C, Limami AM, Vidović M. Carbon and Nitrogen Allocation between the Sink and Source Leaf Tissue in Response to the Excess Excitation Energy Conditions. International Journal of Molecular Sciences. 2023; 24(3):2269. https://doi.org/10.3390/ijms24032269
Chicago/Turabian StyleMilić, Dejana, Bojana Živanović, Jelena Samardžić, Nenad Nikolić, Caroline Cukier, Anis M. Limami, and Marija Vidović. 2023. "Carbon and Nitrogen Allocation between the Sink and Source Leaf Tissue in Response to the Excess Excitation Energy Conditions" International Journal of Molecular Sciences 24, no. 3: 2269. https://doi.org/10.3390/ijms24032269
APA StyleMilić, D., Živanović, B., Samardžić, J., Nikolić, N., Cukier, C., Limami, A. M., & Vidović, M. (2023). Carbon and Nitrogen Allocation between the Sink and Source Leaf Tissue in Response to the Excess Excitation Energy Conditions. International Journal of Molecular Sciences, 24(3), 2269. https://doi.org/10.3390/ijms24032269