Plug-and-Play Lymph Node-on-Chip: Secondary Tumor Modeling by the Combination of Cell Spheroid, Collagen Sponge and T-Cells
Abstract
:1. Introduction
2. Results and Discussion
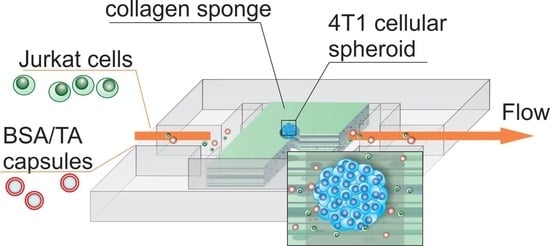
2.1. Design and Fabrication of LNOC
2.2. Study of the Selectivity of Particle Accumulation on LNOC
3. Materials and Methods
3.1. Materials
3.2. Preparation of CaCO3 Particles
3.3. Capsules Preparation
3.4. Capsules Characterisation
3.5. Microfluidic Chip Fabrication
3.6. Formation of the Collagen Sponge—Model of Lymphoid Tissue
3.7. Cell Cultivation
3.8. Formation of Tissue Spheroids
3.9. Fluorescence Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ronaldson-Bouchard, K.; Vunjak-Novakovic, G. Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell Stem Cell 2018, 22, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Peter Guengerich, F. Mechanisms of drug toxicity and relevance to pharmaceutical development. Drug Metab. Pharmacokinet. 2011, 26, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, A.D.; Kuan, V.; Finan, C.; Kruger, F.A.; Gaulton, A.; Chopade, S.; Sofat, R.; MacAllister, R.J.; Overington, J.P.; Hemingway, H.; et al. Improving the odds of drug development success through human genomics: Modelling study. Sci. Rep. 2019, 9, 18911. [Google Scholar] [CrossRef]
- Shanti, A.; Hallfors, N.; Petroianu, G.A.; Planelles, L.; Stefanini, C. Lymph Nodes-On-Chip: Promising Immune Platforms for Pharmacological and Toxicological Applications. Front. Pharmacol. 2021, 12, 711307. [Google Scholar] [CrossRef]
- Nam, K.H.; Smith, A.S.T.; Lone, S.; Kwon, S.; Kim, D.H. Biomimetic 3D Tissue Models for Advanced High-Throughput Drug Screening. J. Lab. Autom. 2015, 20, 201–215. [Google Scholar] [CrossRef]
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue engineering approaches in the design of healthy and pathological in vitro tissue models. Front. Bioeng. Biotechnol. 2017, 5, 40. [Google Scholar] [CrossRef]
- Mandrycky, C.; Phong, K.; Zheng, Y. Tissue engineering toward organ-specific regeneration and disease modeling. MRS Commun. 2017, 7, 332–347. [Google Scholar] [CrossRef]
- Abalymov, A.; Parakhonskiy, B.; Skirtach, A.G. Polymer-and hybrid-based biomaterials for interstitial, connective, vascular, nerve, visceral and musculoskeletal tissue engineering. Polymers 2020, 12, 620. [Google Scholar] [CrossRef]
- Sarvazyan, N.; Exercises, P. Tissue Engineering Principles, Protocols, and Practical Exercises; Springer Nature: Geneve, Switzerland, 2020; ISBN 9783030396978. [Google Scholar]
- Parfenov, V.A.; Koudan, E.V.; Bulanova, E.A.; Karalkin, P.A.; Pereira, F.D.; Norkin, N.E.; Knyazeva, A.D.; Gryadunova, A.A.; Petrov, O.F.; Vasiliev, M.M.; et al. Scaffold-free, label-free and nozzle-free biofabrication technology using magnetic levitational assembly. Biofabrication 2018, 10, 034104. [Google Scholar] [CrossRef]
- Belair, D.G.; Le, N.N.; Murphy, W.L. Design of growth factor sequestering biomaterials. Chem. Commun. 2014, 50, 15651–15668. [Google Scholar] [CrossRef] [PubMed]
- Abalymov, A.; Van Poelvoorde, L.; Atkin, V.; Skirtach, A.G.; Konrad, M.; Parakhonskiy, B. Alkaline Phosphatase Delivery System Based on Calcium Carbonate Carriers for Acceleration of Ossification. ACS Appl. Bio Mater. 2020, 3, 2986–2996. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.S.; Yesantharao, P.; Huang, C.Y.; Mattson, G.; Boktor, J.; Fukunishi, T.; Zhang, H.; Hibino, N. 3D bioprinting using stem cells. Pediatr. Res. 2018, 83, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Vidiasheva, I.V.; Abalymov, A.A.; Kurochkin, M.A.; Mayorova, O.A.; Lomova, M.V.; German, S.V.; Khalenkow, D.N.; Zharkov, M.N.; Gorin, D.A.; Skirtach, A.G.; et al. Transfer of cells with uptaken nanocomposite, magnetite-nanoparticle functionalized capsules with electromagnetic tweezers. Biomater. Sci. 2018, 6, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- De Moor, L.; Fernandez, S.; Vercruysse, C.; Tytgat, L.; Asadian, M.; De Geyter, N.; Van Vlierberghe, S.; Dubruel, P.; Declercq, H. Hybrid Bioprinting of Chondrogenically Induced Human Mesenchymal Stem Cell Spheroids. Front. Bioeng. Biotechnol. 2020, 8, 484. [Google Scholar] [CrossRef]
- Shanti, A.; Teo, J.; Stefanini, C. In vitro immune organs-on-chip for drug development: A review. Pharmaceutics 2018, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Huang, C.; Lin, Y.D.; Ivanovskaya, A.N.; O’Hara, T.J.; Booth, R.H.; Creek, C.J.; Enright, H.A.; Soscia, D.A.; Belle, A.M.; et al. Simultaneous electrical recording of cardiac electrophysiology and contraction on chip. Lab Chip 2017, 17, 1732–1739. [Google Scholar] [CrossRef]
- Vernetti, L.A.; Senutovitch, N.; Boltz, R.; DeBiasio, R.; Ying Shun, T.; Gough, A.; Taylor, D.L. A human liver microphysiology platform for investigating physiology, drug safety, and disease models. Exp. Biol. Med. 2016, 241, 101–114. [Google Scholar] [CrossRef]
- Hassell, B.A.; Goyal, G.; Lee, E.; Sontheimer-Phelps, A.; Levy, O.; Chen, C.S.; Ingber, D.E. Human Organ Chip Models Recapitulate Orthotopic Lung Cancer Growth, Therapeutic Responses, and Tumor Dormancy In Vitro. Cell Rep. 2017, 21, 508–516. [Google Scholar] [CrossRef]
- Jalili-Firoozinezhad, S.; Prantil-Baun, R.; Jiang, A.; Potla, R.; Mammoto, T.; Weaver, J.C.; Ferrante, T.C.; Kim, H.J.; Cabral, J.M.S.; Levy, O.; et al. Modeling radiation injury-induced cell death and countermeasure drug responses in a human Gut-on-a-Chip article. Cell Death Dis. 2018, 9, 223. [Google Scholar] [CrossRef] [Green Version]
- Greenlee, J.D.; King, M.R. Engineered fluidic systems to understand lymphatic cancer metastasis. Biomicrofluidics 2020, 14, 011502. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Soon, R.H.; Lim, C.T.; Khoo, B.L.; Han, J. Microfluidic modelling of the tumor microenvironment for anti-cancer drug development. Lab Chip 2019, 19, 369–386. [Google Scholar] [CrossRef]
- Boussommier-Calleja, A.; Li, R.; Chen, M.B.; Wong, S.C.; Kamm, R.D. Microfluidics: A New Tool for Modeling Cancer–Immune Interactions. Trends Cancer 2016, 2, 6–19. [Google Scholar] [CrossRef]
- Sleeboom, J.J.F.; Amirabadi, H.E.; Nair, P.; Sahlgren, C.M.; Den Toonder, J.M.J. Metastasis in context: Modeling the tumor microenvironment with cancer-on-a-chip approaches. DMM Dis. Model. Mech. 2018, 11, dmm033100. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Sontheimer-Phelps, A.; Hassell, B.A.; Ingber, D.E. Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer 2019, 19, 65–81. [Google Scholar] [CrossRef]
- Zhou, H.; Lei, P.J.; Padera, T.P. Progression of metastasis through lymphatic system. Cells 2021, 10, 627. [Google Scholar] [CrossRef]
- Carlo, J.T.; Grant, M.D.; Knox, S.M.; Jones, R.C.; Hamilton, C.S.; Livingston, S.A.; Kuhn, J.A. Survival Analysis Following Sentinel Lymph Node Biopsy: A Validation Trial Demonstrating Its Accuracy in Staging Early Breast Cancer. Baylor Univ. Med. Cent. Proc. 2005, 18, 103–107. [Google Scholar] [CrossRef]
- Porto Pinheiro, L.G.; Maia, M.; Rocha, J.I.X.; Cruz, D.S. Sentinel Lymph Node Biopsy: Actual Topics. Actas Dermosifiliogr. 2019, 110, 425. [Google Scholar]
- Elgqvist, J. Nanoparticles as theranostic vehicles in experimental and clinical applications-focus on prostate and breast cancer. Int. J. Mol. Sci. 2017, 18, 1102. [Google Scholar] [CrossRef]
- Giese, C.; Demmler, C.D.; Ammer, R.; Hartmann, S.; Lubitz, A.; Miller, L.; Müller, R.; Marx, U. A human lymph node in vitro—Challenges and progress. Artif. Organs 2006, 30, 803–808. [Google Scholar] [CrossRef]
- Moura Rosa, P.; Gopalakrishnan, N.; Ibrahim, H.; Haug, M.; Halaas, Ø. The intercell dynamics of T cells and dendritic cells in a lymph node-on-a-chip flow device. Lab Chip 2016, 16, 3728–3740. [Google Scholar] [CrossRef]
- Hospodiuk-Karwowski, M.; Chi, K.; Pritchard, J.; Catchmark, J.M. Vascularized pancreas-on-a-chip device produced using a printable simulated extracellular matrix. Biomed. Mater. 2022, 7, 065006. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Yuan Hsin, H.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef]
- Arlk, Y.B.; De Sa Vivas, A.; Laarveld, D.; Van Laar, N.; Gemser, J.; Visscher, T.; Van Den Berg, A.; Passier, R.; Van Der Meer, A.D. Collagen i based enzymatically degradable membranes for organ-on-a-chip barrier models. ACS Biomater. Sci. Eng. 2021, 7, 2998–3005. [Google Scholar]
- Trevaskis, N.L.; Lee, G.; Escott, A.; Phang, K.L.; Hong, J.; Cao, E.; Katneni, K.; Charman, S.A.; Han, S.; Charman, W.N.; et al. Intestinal Lymph Flow, and Lipid and Drug Transport Scale Allometrically from Pre-clinical Species to Humans. Front. Physiol. 2020, 11, 458. [Google Scholar] [CrossRef]
- Euscher, E. Pathology of sentinel lymph nodes: Historical perspective and current applications in gynecologic cancer. Int. J. Gynecol. Cancer 2020, 30, 394–401. [Google Scholar] [CrossRef]
- Ramirez, A.; Merwitz, B.; Lee, H.; Vaughan, E.; Maisel, K. Multiple particle tracking using PEGylated nanoparticles reveals lymph node is riddled with heterogeneous pores smaller than reported and interstitial tissue material properties vary by lymph node location. bioRxiv 2022. [Google Scholar] [CrossRef]
- Jia, L.; Xie, Z.; Zheng, J.; Liu, L.; He, Y.; Liu, F.; He, Y. Morphological Studies of Lymphatic Labyrinths in the Rat Mesenteric Lymph Node. Anat. Rec. 2012, 295, 1291–1301. [Google Scholar] [CrossRef]
- Lin, H.J.; Wang, W.; Huang, Y.Y.; Liao, W.T.; Lin, T.Y.; Lin, S.Y.; Liu, D.Z. Decellularized lymph node scaffolding as a carrier for dendritic cells to induce anti-tumor immunity. Pharmaceutics 2019, 11, 553. [Google Scholar] [CrossRef]
- Kan, Y.; Cvjetinovic, J.; Statnik, E.S.; Ryazantsev, S.V.; Anisimova, N.Y.; Kiselevskiy, M.V.; Salimon, A.I.; Maksimkin, A.V.; Korsunsky, A.M. The fabrication and characterization of bioengineered ultra-high molecular weight polyethylene-collagen-hap hybrid bone-cartilage patch. Mater. Today Commun. 2020, 24, 101052. [Google Scholar] [CrossRef]
- Wegst, U.G.K.; Schecter, M.; Donius, A.E.; Hunger, P.M. Biomaterials by freeze casting. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 2099–2121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anada, T.; Fukuda, J.; Sai, Y.; Suzuki, O. An oxygen-permeable spheroid culture system for the prevention of central hypoxia and necrosis of spheroids. Biomaterials 2012, 33, 8430–8441. [Google Scholar] [CrossRef] [PubMed]
- Masiello, T.; Dhall, A.; Hemachandra, L.P.M.; Tokranova, N.; Melendez, J.A.; Castracane, J. A dynamic culture method to produce ovarian cancer spheroids under physiologically-relevant shear stress. Cells 2018, 7, 277. [Google Scholar] [CrossRef] [PubMed]
- DeStefano, J.G.; Williams, A.; Wnorowski, A.; Yimam, N.; Searson, P.C.; Wong, A.D. Real-time quantification of endothelial response to shear stress and vascular modulators. Integr. Biol. 2017, 9, 362–374. [Google Scholar] [CrossRef]
- Lu, H.; Stenzel, M.H. Multicellular Tumor Spheroids (MCTS) as a 3D In Vitro Evaluation Tool of Nanoparticles. Small 2018, 14, 1702858. [Google Scholar] [CrossRef]
- Anisimov, R.A.; Gorin, D.A.; Abalymov, A.A. 3D Cell Spheroids as A Tool for Evaluating the Effectiveness of Carbon Nanotubes as A Drug Delivery and Photothermal Therapy Agents. C 2022, 8, 56. [Google Scholar] [CrossRef]
- Novoselova, M.V.; German, S.V.; Sindeeva, O.A.; Kulikov, O.A.; Minaeva, O.V.; Brodovskaya, E.P.; Ageev, V.P.; Zharkov, M.N.; Pyataev, N.A.; Sukhorukov, G.B.; et al. Submicron-Sized Nanocomposite Magnetic-Sensitive Carriers: Controllable Organ Distribution and Biological Effects. Polymers 2019, 11, 1082. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Petrov, A.I.; Prevot, M.; Sukhorukov, G.B. Matrix Polyelectrolyte Microcapsules: New System for Macromolecule Encapsulation. Langmuir 2004, 20, 3398–3406. [Google Scholar] [CrossRef]
- German, S.V.; Novoselova, M.V.; Bratashov, D.N.; Demina, P.A.; Atkin, V.S.; Voronin, D.V.; Khlebtsov, B.N.; Parakhonskiy, B.V.; Sukhorukov, G.B.; Gorin, D.A. High-efficiency freezing-induced loading of inorganic nanoparticles and proteins into micron- and submicron-sized porous particles. Sci. Rep. 2018, 8, 17763. [Google Scholar] [CrossRef]
- Ma, B.; Jablonska, J.; Lindenmaier, W.; Dittmar, K.E.J. Immunohistochemical study of the reticular and vascular network of mouse lymph node using vibratome sections. Acta Histochem. 2007, 109, 15–28. [Google Scholar] [CrossRef]
- Nong, L.M.; Zhou, D.; Zheng, D.; Jiang, Y.Q.; Xu, N.W.; Zhao, G.Y.; Wei, H.; Zhou, S.Y.; Han, H.; Han, L. The effect of different cross-linking conditions of EDC/NHS on type II collagen scaffolds: An in vitro evaluation. Cell Tissue Bank. 2019, 20, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.; Johal, R.K.; Hamaia, S.W.; Best, S.M.; Cameron, R.E. Tunable bioactivity and mechanics of collagen-based tissue engineering constructs: A comparison of EDC-NHS, genipin and TG2 crosslinkers. Biomaterials 2020, 254, 120109. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
German, S.V.; Abalymov, A.A.; Kurochkin, M.A.; Kan, Y.; Gorin, D.A.; Novoselova, M.V. Plug-and-Play Lymph Node-on-Chip: Secondary Tumor Modeling by the Combination of Cell Spheroid, Collagen Sponge and T-Cells. Int. J. Mol. Sci. 2023, 24, 3183. https://doi.org/10.3390/ijms24043183
German SV, Abalymov AA, Kurochkin MA, Kan Y, Gorin DA, Novoselova MV. Plug-and-Play Lymph Node-on-Chip: Secondary Tumor Modeling by the Combination of Cell Spheroid, Collagen Sponge and T-Cells. International Journal of Molecular Sciences. 2023; 24(4):3183. https://doi.org/10.3390/ijms24043183
Chicago/Turabian StyleGerman, Sergei V., Anatolii A. Abalymov, Maxim A. Kurochkin, Yuliya Kan, Dmitry A. Gorin, and Marina V. Novoselova. 2023. "Plug-and-Play Lymph Node-on-Chip: Secondary Tumor Modeling by the Combination of Cell Spheroid, Collagen Sponge and T-Cells" International Journal of Molecular Sciences 24, no. 4: 3183. https://doi.org/10.3390/ijms24043183
APA StyleGerman, S. V., Abalymov, A. A., Kurochkin, M. A., Kan, Y., Gorin, D. A., & Novoselova, M. V. (2023). Plug-and-Play Lymph Node-on-Chip: Secondary Tumor Modeling by the Combination of Cell Spheroid, Collagen Sponge and T-Cells. International Journal of Molecular Sciences, 24(4), 3183. https://doi.org/10.3390/ijms24043183