The Discovery of Ribosomal Protein bL31 from Escherichia coli: A Long Story Revisited
Abstract
:1. Introduction
1.1. Separation of Ribosomal Proteins (r-Proteins) Using 2D PAGE
1.2. Improvement of the 2D PAGE Method
2. Characterization of bL131 and Its Truncated Form
2.1. Detection of Two Spots Corresponding to bL31
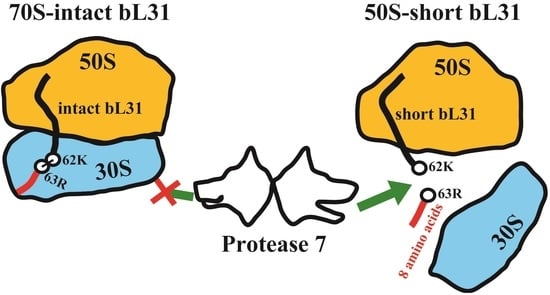
2.2. Intact bL31 Is Cleaved by Protease 7
2.3. Localization of bL31 on the Ribosome
2.4. The Effect of Protease 7 on the Subunit Association of Ribosomes Prepared Using the Conventional Extraction Method
2.5. The Protein Levels of Intact and Short bL31s in the 70S and 50S Fractions
2.6. The Effect of bL31 on the Translational Activity
2.7. bL31 Is Involved in Hibernation
2.8. YkgM, Which Is a bL31 Paralog, Maintains Translation Activity by Replacing bL31 When It Defects
2.9. The Effect of bL31 Function on Bacterial Growth
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaltschmidt, E.; Wittmann, H.G. Ribosomal proteins, XII. Number of proteins in small and large subunits of Escherichia coli as determined by two-dimensional gel electrophoresis. Proc. Natl. Acad. Sci. USA 1970, 67, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Wada, A. Analysis of Escherichia coli ribosomal proteins by an improved two-dimensional gel electrophoresis. I. Detection of four new proteins. J. Biochem. 1986, 100, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Wada, A. Analysis of Escherichia coli ribosomal proteins by an improved two-dimensional gel electrophoresis. II. Characterization of four new proteins. J. Biochem. 1986, 100, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Hardy, S.J.; Kurland, C.G.; Voynow, P.; Mora, G. The ribosomal proteins of Escherichia coli. I. Purification of 30S ribosomal proteins. Biochemistry 1969, 8, 2897–2905. [Google Scholar] [CrossRef] [PubMed]
- Ueta, M.; Wada, C.; Wada, A. Formation of 100S ribosomes in Staphylococcus aureus by the hibernation promoting factor homolog SaHPF. Genes Cells 2010, 15, 43–58. [Google Scholar] [CrossRef]
- Wada, A.; Yamazaki, Y.; Fujita, N.; Ishihama, A. Structure and probable genetic location of a “ribosome modulation factor” associated with 100S ribosomes in stationary phase Escherichia coli cells. Proc. Natl. Acad. Sci. USA 1990, 87, 2657–2661. [Google Scholar] [CrossRef]
- Plunkett, G., III; Burland, V.; Daniels, D.L.; Blattner, F.R. Analysis of the Escherichia coli genome. III. DNA sequence of the region from 87.2 to 89.2 minutes. Nucleic Acids Res. 1993, 21, 3391–3398. [Google Scholar] [CrossRef]
- Wada, A. Growth phase coupled modulation of Escherichia coli ribosomes. Genes Cells 1998, 3, 203–208. [Google Scholar] [CrossRef]
- Arnold, R.J.; Reilly, J.P. Observation of Escherichia coli Ribosomal Proteins and Their Posttranslational Modifications by Mass Spectrometry. Anal. Biochem. 1999, 269, 105–112. [Google Scholar] [CrossRef]
- Eistetter, A.J.; Butler, P.D.; Traut, R.R.; Fanning, T.G. Characterization of Escherichia coli 50S ribosomal protein L31. FEMS Microbiol. Lett. 1999, 180, 345–349. [Google Scholar] [CrossRef]
- Tüting, C.; Lacobucci, C.; Ihling, C.H.; Kastritis, P.L.; Sinz, A. Structural analysis of 70S ribosomes by cross-linking/mass spectrometry reveals conformational plasticity. Sci. Rep. 2020, 10, 12618. [Google Scholar] [CrossRef]
- Lammi, M.; Pon, C.L.; Gualerzi, C.O. The NH2-terminal cleavage of Escherichia coli translational initiation factor IF3 A mechanism to control the intracellular level of the factor? FEBS Lett. 1987, 215, 115–121. [Google Scholar] [CrossRef]
- Lassen, S.F.; Mortensen, K.K.; Sperling-Petersen, H.U. OmpT proteolysis of E. coli initiation factor IF2, elimination of a cleavage site by site directed mutagenesis. Biochem. Int. 1992, 27, 601–611. [Google Scholar]
- Milon, P.; Konevega, A.L.; Peske, F.; Fabbretti, A.; Guaerzi, C.O.; Rodnina, M.V. Transient Kinetics, Flurescence, and FRET in Studies of Initiation of Translation in Bacteria. Meth. Enzymol. 2007, 430, 1–30. [Google Scholar] [CrossRef]
- Gao, H.; Sengupta, J.; Valle, M.; Korostelev, A.; Eswar, N.; Stagg, S.M.; Roey, P.V.; Agrawal, R.K.; Harvey, S.C.; Sali, A.; et al. Study of the structural dynamics of the E. coli 70S ribosome using real-space refinement. Cell 2003, 113, 789–801. [Google Scholar] [CrossRef]
- Fanning, T.; Traut, R. Topography of the E. coli 5S RNA-protein complex as determined by crosslinking with dimethyl suberimidate and dimethyl-3, 3P-dithiobispropionimidate. Nucleic Acids Res. 1981, 9, 993–1004. [Google Scholar] [CrossRef]
- Selmer, M.; Dunham, C.M.; Murphy, F.V.; Weixlbaumer, A.; Petry, S.; Kelley, A.C.; Weir, J.R.; Ramakrishnan, V. Structure of the 70S ribosome complexed with mRNA and tRNA. Science 2006, 313, 1935–1942. [Google Scholar] [CrossRef]
- Jenner, L.; Demeshkina, N.; Yusupova, G.; Yusupov, M. Structural rearrangements of the ribosome at the tRNA proofreading step. Nat. Struct. Mol. Biol. 2010, 17, 1072–1078. [Google Scholar] [CrossRef]
- Shasmal, M.; Chakraborty, B.; Sengupta, J. Intrinsic molecular properties of the protein-protein bridge facilitate ratchet-like motion of the ribosome. Biochem. Biophys. Res. Commun. 2010, 399, 192–197. [Google Scholar] [CrossRef]
- Fischer, N.; Neumann, P.; Konevega, A.L.; Bock, L.V.; Ficner, R.; Rodnina, M.V.; Stark, H. Structure of the E. coli ribosome-EF-Tu complex at <3 Å resolution by Cs-corrected cryo-EM. Nature 2015, 520, 567–570. [Google Scholar] [CrossRef]
- Lilleorg, S.; Reier, K.; Remme, J.; Liiv, A. The Intersubunit Bridge B1b of the Bacterial Ribosome Facilitates Initiation of Protein Synthesis and Maintenance of Translational Fidelity. J. Mol. Biol. 2017, 429, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Debey, P.; Hui Bon Hoa, G.; Douzou, P.; Godefroy-Colburn, T.; Graffe, M.; Grunberg-Manago, M. Ribosomal subunit interaction as studied by light scattering. Evidence of different classes of ribosome preparations. Biochemistry 1975, 14, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Wishnia, A.; Boussert, A.; Graffe, M.; Dessen, P.H.; Grunberg-Manago, M. Kinetics of the reversible association of ribosomal subunits: Stopped-flow studies of the rate law and of the effect of Mg2+. J. Mol. Biol. 1975, 93, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Noll, M.; Noll, H. Structural dynamics of bacterial ribosomes. V. Magnesium-dependent dissociation of tight couples into subunits: Measurements of dissociation constants and exchange rates. J. Mol. Biol. 1976, 105, 111–130. [Google Scholar] [CrossRef]
- Rosano, C.L.; Hurwitz, C. Antagonistic action between spermidine and putrescine on association and dissociation of purified, run-off ribosomes from Escherichia coli. J. Biol. Chem. 1977, 252, 652–654. [Google Scholar] [CrossRef]
- Ueta, M.; Wada, C.; Bessho, Y.; Maeda, M.; Wada, A. Ribosomal protein L31 in Escherichia coli contributes to ribosome subunit association and translation, whereas short L31 cleaved by protease 7 reduces both activities. Genes Cells 2017, 22, 452–471. [Google Scholar] [CrossRef]
- Shimizu, Y.; Kanamori, T.; Ueda, T. Protein synthesis by pure translation systems. Methods 2005, 36, 299–304. [Google Scholar] [CrossRef]
- Ueta, M.; Ohniwa, R.L.; Yoshida, H.; Maki, Y.; Wada, C.; Wada, A. Role of HPF (hibernation promoting factor) in translational activity in Escherichia coli. J. Biochem. 2008, 143, 425–433. [Google Scholar] [CrossRef]
- Maki, Y.; Yoshida, H.; Wada, A. Two proteins, YfiA and YhbH, associated with resting ribosomes in stationary phase Escherichia coli. Genes Cells 2000, 5, 965–974. [Google Scholar] [CrossRef]
- Izutsu, K.; Wada, A.; Wada, C. Expression of ribosome modulation factor (RMF) in Escherichia coli requires ppGpp. Genes Cells 2001, 6, 665–676. [Google Scholar] [CrossRef]
- Yoshida, H.; Maki, Y.; Kato, H.; Fujisawa, H.; Izutsu, K.; Wada, C.; Wada, A. The ribosome modulation factor (RMF) binding site on the 100S ribosome of Escherichia coli. J. Biochem. 2002, 132, 983–989. [Google Scholar] [CrossRef]
- Ueta, M.; Yoshida, H.; Wada, C.; Baba, T.; Mori, H.; Wada, A. Ribosome binding proteins YfiA and YhbH have opposite functions during 100S formation in the stationary phase of Escherichia coli. Genes Cells 2005, 10, 1103–1112. [Google Scholar] [CrossRef]
- Yoshida, H.; Wada, A. The 100S ribosome: Ribosomal hibernation induced by stress. Wiley Interdiscip. Rev. RNA 2014, 5, 723–732. [Google Scholar] [CrossRef]
- Maki, Y.; Yoshida, H. Ribosomal Hibernation-Associated Factors in Escherichia coli. Microorganisms 2021, 10, 33. [Google Scholar] [CrossRef]
- Kato, T.; Yoshida, H.; Miyata, T.; Maki, Y.; Wada, A.; Namba, K. Structure of the 100S ribosome in the hibernation stage revealed by electron cryomicroscopy. Structure 2010, 18, 719–724. [Google Scholar] [CrossRef]
- Ortiz, J.O.; Brandt, F.; Matias, V.R.F.; Sennels, L.; Rappsilber, J.; Scheres, S.H.W.; Eibauer, M.; Hartl, F.U.; Baumeister, W. Structure of hibernating ribosomes studied by cryoelectron tomography in vitro and in situ. J. Cell Biol. 2010, 190, 613–621. [Google Scholar] [CrossRef]
- Ueta, M.; Wada, C.; Daifuku, T.; Sako, Y.; Bessho, Y.; Kitamura, A.; Ohniwa, R.L.; Morikawa, K.; Yoshida, H.; Kato, T.; et al. Conservation of two distinct types of 100S ribosome in bacteria. Genes Cells 2013, 18, 554–574. [Google Scholar] [CrossRef] [PubMed]
- Franken, L.E.; Oostergetel, G.T.; Pijning, T.; Puri, P.; Arkhipova, V.; Boekema, E.J.; Poolman, B.; Guskov, A. A general mechanism of ribosome dimerization revealed by single-particle cryo-electron microscopy. Nat. Commun. 2017, 8, 722. [Google Scholar] [CrossRef] [PubMed]
- Khusainov, I.; Vicens, Q.; Ayupov, R.; Usachev, K.; Myasnikov, A.; Simonetti, A.; Validov, S.; Kieffer, B.; Yusupova, G.; Yusupov, M.; et al. Structures and dynamics of hibernating ribosomes from Staphylococcus aureus mediated by intermolecular interactions of HPF. EMBO J. 2017, 36, 2073–2087. [Google Scholar] [CrossRef] [PubMed]
- Flygaard, R.K.; Boegholm, N.; Yusupov, M.; Jenner, L.B. Cryo-EM structure of the hibernating Thermus thermophilus 100S ribosome reveals a protein-mediated dimerization mechanism. Nat. Commun. 2018, 9, 4179. [Google Scholar] [CrossRef]
- Makarova, K.S.; Ponomarev, V.A.; Koonin, E.V. Two C or not two C: Recurrent disruption of Zn-ribbons, gene duplication, lineage-specific gene loss, and horizontal gene transfer in evolution of bacterial ribosomal proteins. Genome Biol. 2001, 2, research0033.1. [Google Scholar] [CrossRef]
- Panina, E.M.; Mironov, A.A.; Gelfand, M.S. Comparative genomics of bacterial zinc regulons: Enhanced ion transport, pathogenesis, and rearrangement of ribosomal proteins. Proc. Natl. Acad. Sci. USA 2003, 100, 9912–9917. [Google Scholar] [CrossRef]
- Hensley, M.P.; Gunasekera, T.S.; Easton, J.A.; Sigdel, T.K.; Sugarbaker, S.A.; Klingbeil, L.; Breece, R.M.; Tierney, D.L.; Crowder, M.W. Characterization of Zn (II)-responsive ribosomal proteins YkgM and L31 in E. coli. J. Inorg. Biochem. 2012, 111, 164–172. [Google Scholar] [CrossRef]
- Lilleorg, S.; Reier, K.; Pulk, A.; Liiv, A.; Tammsalu, T.; Peil, L.; Cate, J.H.D.; Remme, J. Bacterial ribosome heterogeneity: Changes in ribosomal protein composition during transition into stationary growth phase. Biochimie 2019, 156, 169–180. [Google Scholar] [CrossRef]
- Graham, A.I.; Hunt, S.; Stokes, S.L.; Bramall, N.; Bunch, J.; Cox, A.G.; Cameron, W.M.; Poole, R.K. Severe zinc depletion of Escherichia coli: Roles for high affinity zinc binding by ZinT, zinc transport and zinc-independent proteins. J. Biol. Chem. 2009, 284, 18377–18389. [Google Scholar] [CrossRef]
- Hemm, M.R.; Paul, B.J.; Miranda-Ríos, J.; Zhang, A.; Soltanzad, N.; Storz, G. Small stress response proteins in Escherichia coli: Proteins missed by classical proteomic studies. J. Bacteriol. 2010, 192, 46–58. [Google Scholar] [CrossRef]
- Gabriel, S.E.; Helmann, J.D. Contributions Zur-controlled ribosomal proteins to growth under zinc starvation conditions. J. Bacteriol. 2009, 191, 6116–6122. [Google Scholar] [CrossRef]
- Nanamiya, H.; Akanuma, G.; Natori, Y.; Murayama, R.; Kosono, S.; Kudo, T.; Kobayashi, K.; Ogasawara, N.; Park, S.M.; Ochi, K.; et al. Zinc is a key factor in controlling alternation of two types of L31 protein in the Bacillus subtilis ribosomes. Mol. Microbiol. 2004, 52, 273–283. [Google Scholar] [CrossRef]
- Owen, G.A.; Pascoe, B.; Kallifidas, D.; Paget, M.S. Zinc-responsive regulation of alternative ribosomal protein genes in Streptomyces coelicolor involves Zur and σR. J. Bacteriol. 2007, 189, 4078–4086. [Google Scholar] [CrossRef]
- Shin, J.H.; Helmann, J.D. Molecular logic of the Zur-regulated zinc deprivation response in Bacillus subtilis . Nat. Commun. 2016, 7, 12612. [Google Scholar] [CrossRef]
- Ueta, M.; Wada, C.; Wada, A. YkgM and YkgO maintain translation by replacing their paralogs, zinc-binding ribosomal proteins L31 and L36, with identical activities. Genes Cells 2020, 25, 562–581. [Google Scholar] [CrossRef] [PubMed]
- Agirrezabala, X.; Liao, H.Y.; Schreiner, E.; Fu, J.; Ortiz-Meoz, R.F.; Schulten, K.; Green, R.; Frank, J. Structural characterization of mRNA-tRNA translocation intermediates. Proc. Natl. Acad. Sci. USA 2012, 109, 6094–6099. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wada, A.; Ueta, M.; Wada, C. The Discovery of Ribosomal Protein bL31 from Escherichia coli: A Long Story Revisited. Int. J. Mol. Sci. 2023, 24, 3445. https://doi.org/10.3390/ijms24043445
Wada A, Ueta M, Wada C. The Discovery of Ribosomal Protein bL31 from Escherichia coli: A Long Story Revisited. International Journal of Molecular Sciences. 2023; 24(4):3445. https://doi.org/10.3390/ijms24043445
Chicago/Turabian StyleWada, Akira, Masami Ueta, and Chieko Wada. 2023. "The Discovery of Ribosomal Protein bL31 from Escherichia coli: A Long Story Revisited" International Journal of Molecular Sciences 24, no. 4: 3445. https://doi.org/10.3390/ijms24043445
APA StyleWada, A., Ueta, M., & Wada, C. (2023). The Discovery of Ribosomal Protein bL31 from Escherichia coli: A Long Story Revisited. International Journal of Molecular Sciences, 24(4), 3445. https://doi.org/10.3390/ijms24043445