Neutralising Effects of Different Antibodies on Clostridioides difficile Toxins TcdA and TcdB in a Translational Approach
Abstract
:1. Introduction
2. Results
2.1. Recombinant and Natural sIgA Antibodies Were Successfully Purified
2.2. Recombinant and Natural Antibodies Mainly Bind to TcdB
2.3. Recombinant Antibodies Exhibited Superior TcdB Neutralisation Potential Compared to Natural sIgA Molecules
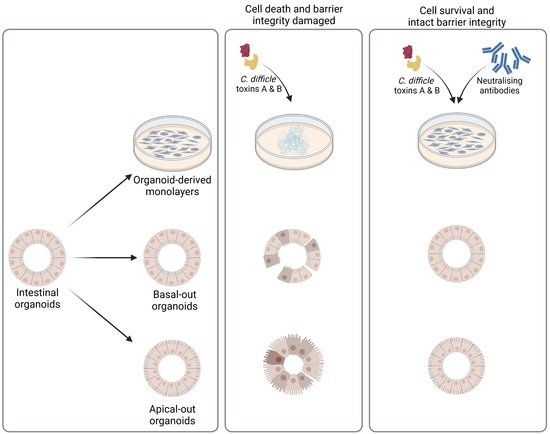
2.4. Successful Establishment of Apical-Out and Floating Basal-Out Organoids
2.5. Considerably Different Effects of TcdA and TcdB on Basal-Out and Apical-Out Organoids
3. Discussion
4. Materials and Methods
4.1. Recombinant Monoclonal sIgA
4.2. Isolation of Caprine Whey sIgA
4.3. ELISA
4.4. Organoid Culture
4.5. Generation of Organoid-Derived Monolayers
4.6. Sulforhodamine B Cytotoxicity Assay
4.7. Generation of Floating Basal-Out and Apical-Out Organoids
4.8. DAPI/Phalloidin Staining
4.9. FITC-Dextran Barrier Integrity Assay
4.10. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johansson, M.E.V.; Sjövall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the Gut Microbiome and Mucosal Immune System. Mil Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Jia, Z. Microbiome modulates intestinal homeostasis against inflammatory diseases. Veter- Immunol. Immunopathol. 2018, 205, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Crockett, S.D.; Shaheen, N.J. The Burden of Gastrointestinal and Liver Disease Around the World. In GI Epidemiology: Diseases and Clinical Methodology: Second Edition; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Honneffer, J.; Minamoto, Y.; Suchodolski, J.S. Microbiota alterations in acute and chronic gastrointestinal inflammation of cats and dogs. World J. Gastroenterol. 2014, 20, 16489–16497. [Google Scholar] [CrossRef] [PubMed]
- Mondo, E.; Marliani, G.; Accorsi, P.A.; Cocchi, M.; Di Leone, A. Role of gut microbiota in dog and cat’s health and diseases. Open Veter- J. 2019, 9, 253–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, A.R.; Proctor, L.M.; Surette, M.G.; Suchodolski, J.S. The Microbiome. Veter- Pathol. 2015, 53, 10–21. [Google Scholar] [CrossRef]
- Rodriguez Diaz, C.; Seyboldt, C.; Rupnik, M.; Non-Human, C. Difficile Reservoirs and Sources: Animals, Food, Environment. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1050, pp. 227–243. [Google Scholar]
- Otten, A.M.; Reid-Smith, R.J.; Fazil, A.; Weese, J.S. Disease transmission model for community-associated Clostridium difficile infection. Epidemiology Infect. 2010, 138, 907–914. [Google Scholar] [CrossRef]
- McLure, A.; Clements, A.C.A.; Kirk, M.; Glass, K. Modelling diverse sources of Clostridium difficile in the community: Importance of animals, infants and asymptomatic carriers. Epidemiology Infect. 2019, 147, e152. [Google Scholar] [CrossRef] [Green Version]
- Redding, L.; Tu, V.; Abbas, A.; Alvarez, M.; Zackular, J.; Gu, C.; Bushman, F.; Kelly, D.; Barnhart, D.; Lee, J.; et al. Genetic and phenotypic characteristics of Clostridium (Clostridioides) difficile from canine, bovine, and pediatric populations. Anaerobe 2022, 74, 102539. [Google Scholar] [CrossRef]
- Corthésy, B. Role of secretory immunoglobulin A and secretory component in the protection of mucosal surfaces. Futur. Microbiol. 2010, 5, 817–829. [Google Scholar] [CrossRef]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. R. Soc. B Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chastant, S.; Mila, H. Passive immune transfer in puppies. Anim. Reprod. Sci. 2019, 207, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Mila, H.; Feugier, A.; Grellet, A.; Anne, J.; Gonnier, M.; Martin, M.; Rossig, L.; Chastant-Maillard, S. Immunoglobulin G concentration in canine colostrum: Evaluation and variability. J. Reprod. Immunol. 2015, 112, 24–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafarzadeh, A.; Sadeghi, M.; Karam, G.A.; Vazirinejad, R. Salivary IgA and IgE levels in healthy subjects: Relation to age and gender. Braz. Oral Res. 2010, 24, 21–27. [Google Scholar] [CrossRef]
- Kerr, M.A.; Mazengera, R.L.; Stewart, W.W. Structure and function of immunoglobulin A receptors on phagocytic cells. Biochem. Soc. Trans. 1990, 18, 215–217. [Google Scholar] [CrossRef] [Green Version]
- Dallas, S.D.; Rolfe, R.D. Binding of Clostridium difficile toxin A to human milk secretory component. J. Med. Microbiol. 1998, 47, 879–888. [Google Scholar] [CrossRef] [Green Version]
- Matlschweiger, A.; Himmler, G.; Linhart, C.; Harasek, M.; Hahn, R. A nonchromatographic process for purification of secretory immunoglobulins from caprine whey. Biotechnol. Prog. 2017, 33, 642–653. [Google Scholar] [CrossRef]
- Matlschweiger, A.; Engelmaier, H.; Himmler, G.; Hahn, R. Secretory immunoglobulin purification from whey by chromatographic techniques. J. Chromatogr. B 2017, 1060, 53–62. [Google Scholar] [CrossRef]
- Van Dissel, J.T.; De Groot, N.; Hensgens, C.M.; Numan, S.; Kuijper, E.J.; Veldkamp, P.; Wout, J.V. Bovine antibody-enriched whey to aid in the prevention of a relapse of Clostridium difficile-associated diarrhoea: Preclinical and preliminary clinical data. J. Med. Microbiol. 2005, 54, 197–205. [Google Scholar] [CrossRef]
- Gustafsson, A.; Kacskovics, I.; Breimer, M.E.; Hammarström, L.; Holgersson, J. Carbohydrate phenotyping of human and animal milk glycoproteins. Glycoconj. J. 2005, 22, 109–118. [Google Scholar] [CrossRef]
- Kim, K.; Pickering, L.K.; DuPont, H.L.; Sullivan, N.; Wilkins, T. In Vitro and in Vivo Neutralizing Activity of Human Colostrum and Milk Against Purified Toxins A and B of Clostridium difficile. J. Infect. Dis. 1984, 150, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Prechtl, C. Potential of Goat Milk SIgA to Neutralize Bacterial Toxins; University of Veterinary Medicine: Vienna, Austria, 2013. [Google Scholar]
- Jiminez, J.A.; Uwiera, T.C.; Inglis, G.D.; Uwiera, R.R.E. Animal models to study acute and chronic intestinal inflammation in mammals. Gut Pathog. 2015, 7, 1–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerquetella, M.; Spaterna, A.; Laus, F.; Tesei, B.; Rossi, G.; Antonelli, E.; Villanacci, V.; Bassotti, G. Inflammatory bowel disease in the dog: Differences and similarities with humans. World J. Gastroenterol. 2010, 16, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Csukovich, G.; Pratscher, B.; Burgener, I.A. The World of Organoids: Gastrointestinal Disease Modelling in the Age of 3R and One Health with Specific Relevance to Dogs and Cats. Animals 2022, 12, 2461. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.; Pratscher, B.; Meneses, A.M.C.; Tschulenk, W.; Walter, I.; Swoboda, A.; Kruitwagen, H.S.; Schneeberger, K.; Penning, L.C.; Spee, B.; et al. Generation of Differentiating and Long-Living Intestinal Organoids Reflecting the Cellular Diversity of Canine Intestine. Cells 2020, 9, 822. [Google Scholar] [CrossRef] [Green Version]
- Jarmo, O.; Veli-Jukka, A.; Eero, M. Treatment of Clostridioides (Clostridium) Difficile Infection. Ann. Med. 2020, 52, 12–20. [Google Scholar] [CrossRef]
- van Prehn, J.; Reigadas, E.; Vogelzang, E.H.; Bouza, E.; Hristea, A.; Guery, B.; Krutova, M.; Norén, T.; Allerberger, F.; Coia, J.E.; et al. European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin. Microbiol. Infect. 2021, 27 (Suppl. S2), S1–S21. [Google Scholar] [CrossRef]
- Bhaskara, V.; Leal, M.T.; Seigner, J.; Friedrich, T.; Kreidl, E.; Gadermaier, E.; Tesarz, M.; Rogalli, A.; Stangl, L.; Wallwitz, J.; et al. Efficient production of recombinant secretory IgA against Clostridium difficile toxins in CHO-K1 cells. J. Biotechnol. 2021, 331, 1–13. [Google Scholar] [CrossRef]
- Nash, T.J.; Morris, K.M.; Mabbott, N.A.; Vervelde, L. Inside-out chicken enteroids with leukocyte component as a model to study host–pathogen interactions. Commun. Biol. 2021, 4, 377. [Google Scholar] [CrossRef]
- Lim, S.C.; Knight, D.R.; Riley, T.V. Clostridium Difficile and One Health. Clin. Microbiol. Infect. 2020, 26, 857–863. [Google Scholar] [CrossRef]
- Sheth, P.M.; Douchant, K.; Uyanwune, Y.; Larocque, M.; Anantharajah, A.; Borgundvaag, E.; Dales, L.; McCreight, L.; McNaught, L.; Moore, C.; et al. Evidence of transmission of Clostridium difficile in asymptomatic patients following admission screening in a tertiary care hospital. PLoS ONE 2019, 14, e0207138. [Google Scholar] [CrossRef]
- Halstead, F.; Ravi, A.; Thomson, N.; Nuur, M.; Hughes, K.; Brailey, M.; Oppenheim, B. Whole genome sequencing of toxigenic Clostridium difficile in asymptomatic carriers: Insights into possible role in transmission. J. Hosp. Infect. 2019, 102, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S. Clostridium (Clostridioides) difficile in animals. J. Veter- Diagn. Investig. 2020, 32, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Finsterwalder, S.; Loncaric, I.; Cabal, A.; Szostak, M.; Barf, L.; Marz, M.; Allerberger, F.; Burgener, I.; Tichy, A.; Feßler, A.; et al. Dogs as carriers of virulent and resistant genotypes of Clostridioides difficile. Zoonoses Public Heal. 2022, 69, 673–681. [Google Scholar] [CrossRef]
- Olson, A.; Diebel, L.N.; Liberati, D.M. Effect of host defenses on Clostridium difficile toxin–induced intestinal barrier injury. J. Trauma: Inj. Infect. Crit. Care 2013, 74, 983–990. [Google Scholar] [CrossRef]
- Voth, D.E.; Ballard, J.D. Clostridium difficile Toxins: Mechanism of Action and Role in Disease. Clin. Microbiol. Rev. 2005, 18, 247–263. [Google Scholar] [CrossRef] [Green Version]
- Williamson, I.A.; Arnold, J.W.; Samsa, L.A.; Gaynor, L.; DiSalvo, M.; Cocchiaro, J.L.; Carroll, I.; Azcarate-Peril, M.A.; Rawls, J.F.; Allbritton, N.L.; et al. A High-Throughput Organoid Microinjection Platform to Study Gastrointestinal Microbiota and Luminal Physiology. Cell Mol. Gastroenterol. Hepatol. 2018, 6, 301–319. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Mslati, M.A.; Davies, E.; Chen, Y.; Allaire, J.M.; Vallance, B.A. Creating a More Perfect Union: Modeling Intestinal Bacteria-Epithelial Interactions Using Organoids. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 769–782. [Google Scholar] [CrossRef]
- Tao, L.; Tian, S.; Zhang, J.; Liu, Z.; Robinson-McCarthy, L.; Miyashita, S.-I.; Breault, D.T.; Gerhard, R.; Oottamasathien, S.; Whelan, S.P.J.; et al. Sulfated glycosaminoglycans and low-density lipoprotein receptor contribute to Clostridium difficile toxin A entry into cells. Nat. Microbiol. 2019, 4, 1760–1769. [Google Scholar] [CrossRef]
- Olling, A.; Goy, S.; Hoffmann, F.; Tatge, H.; Just, I.; Gerhard, R. The Repetitive Oligopeptide Sequences Modulate Cytopathic Potency but Are Not Crucial for Cellular Uptake of Clostridium difficile Toxin A. PLoS ONE 2011, 6, e17623. [Google Scholar] [CrossRef]
- Henkel, D.; Tatge, H.; Schöttelndreier, D.; Tao, L.; Dong, M.; Gerhard, R. Receptor Binding Domains of TcdB from Clostridioides difficile for Chondroitin Sulfate Proteoglycan-4 and Frizzled Proteins Are Functionally Independent and Additive. Toxins 2020, 12, 736. [Google Scholar] [CrossRef] [PubMed]
- Engevik, M.A.; Danhof, H.A.; Chang-Graham, A.L.; Spinler, J.K.; Engevik, K.A.; Herrmann, B.; Endres, B.T.; Garey, K.W.; Hyser, J.M.; Britton, R.A.; et al. Human intestinal enteroids as a model of Clostridioides difficile-induced enteritis. Am. J. Physiol. Liver Physiol. 2020, 318, G870–G888. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Ückert, A.; Landenberger, M.; Papatheodorou, P.; Hoffmann-Richter, C.; Mittler, A.; Ziener, U.; Hägele, M.; Schwan, C.; Müller, M.; et al. Human peptide α-defensin-1 interferes with Clostridioides difficile toxins TcdA, TcdB, and CDT. FASEB J. 2020, 34, 6244–6261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Savidge, T.; Feng, H. The Enterotoxicity of Clostridium difficile Toxins. Toxins 2010, 2, 1848–1880. [Google Scholar] [CrossRef] [Green Version]
- Yuan, P.; Zhang, H.; Cai, C.; Zhu, S.; Zhou, Y.; Yang, X.; He, R.; Li, C.; Guo, S.; Li, S.; et al. Chondroitin sulfate proteoglycan 4 functions as the cellular receptor for Clostridium difficile toxin B. Cell Res. 2014, 25, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Tao, L.; Zhang, J.; Meraner, P.; Tovaglieri, A.; Wu, X.; Gerhard, R.; Zhang, X.; Stallcup, W.B.; Miao, J.; He, X.; et al. Frizzled proteins are colonic epithelial receptors for C. difficile toxin B. Nature 2016, 538, 350–355. [Google Scholar] [CrossRef] [Green Version]
- LaFrance, M.E.; Farrow, M.A.; Chandrasekaran, R.; Sheng, J.; Rubin, D.H.; Lacy, D.B. Identification of an epithelial cell receptor responsible for Clostridium difficile TcdB-induced cytotoxicity. Proc. Natl. Acad. Sci. USA 2015, 112, 7073–7078. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Tao, L.; Wang, T.; Zhang, J.; He, A.; Lam, K.-H.; Liu, Z.; He, X.; Perry, K.; Dong, M.; et al. Structural basis for recognition of frizzled proteins by Clostridium difficile toxin B. Science 2018, 360, 664–669. [Google Scholar] [CrossRef] [Green Version]
- Terada, N.; Ohno, N.; Murata, S.; Katoh, R.; Stallcup, W.B.; Ohno, S. Immunohistochemical study of NG2 chondroitin sulfate proteoglycan expression in the small and large intestines. Histochem. 2006, 126, 483–490. [Google Scholar] [CrossRef]
- Petersen, L.; Stroh, S.; Schöttelndreier, D.; Grassl, G.A.; Rottner, K.; Brakebusch, C.; Fahrer, J.; Genth, H. The Essential Role of Rac1 Glucosylation in Clostridioides difficile Toxin B-Induced Arrest of G1-S Transition. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Co, J.Y.; Margalef-Català, M.; Li, X.; Mah, A.T.; Kuo, C.J.; Monack, D.M.; Amieva, M.R. Controlling Epithelial Polarity: A Human Enteroid Model for Host-Pathogen Interactions. Cell Rep. 2019, 26, 2509–2520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardenbacher, M.; Ruder, B.; Britzen-Laurent, N.; Naschberger, E.; Becker, C.; Palmisano, R.; Stürzl, M.; Tripal, P. Investigating Intestinal Barrier Breakdown in Living Organoids. J. Vis. Exp. 2020, 157, e60546. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csukovich, G.; Kramer, N.; Pratscher, B.; Gotic, I.; Freund, P.; Hahn, R.; Himmler, G.; Brandt, S.; Burgener, I.A. Neutralising Effects of Different Antibodies on Clostridioides difficile Toxins TcdA and TcdB in a Translational Approach. Int. J. Mol. Sci. 2023, 24, 3867. https://doi.org/10.3390/ijms24043867
Csukovich G, Kramer N, Pratscher B, Gotic I, Freund P, Hahn R, Himmler G, Brandt S, Burgener IA. Neutralising Effects of Different Antibodies on Clostridioides difficile Toxins TcdA and TcdB in a Translational Approach. International Journal of Molecular Sciences. 2023; 24(4):3867. https://doi.org/10.3390/ijms24043867
Chicago/Turabian StyleCsukovich, Georg, Nina Kramer, Barbara Pratscher, Ivana Gotic, Patricia Freund, Rainer Hahn, Gottfried Himmler, Sabine Brandt, and Iwan Anton Burgener. 2023. "Neutralising Effects of Different Antibodies on Clostridioides difficile Toxins TcdA and TcdB in a Translational Approach" International Journal of Molecular Sciences 24, no. 4: 3867. https://doi.org/10.3390/ijms24043867
APA StyleCsukovich, G., Kramer, N., Pratscher, B., Gotic, I., Freund, P., Hahn, R., Himmler, G., Brandt, S., & Burgener, I. A. (2023). Neutralising Effects of Different Antibodies on Clostridioides difficile Toxins TcdA and TcdB in a Translational Approach. International Journal of Molecular Sciences, 24(4), 3867. https://doi.org/10.3390/ijms24043867