Extracellular Vesicle-Based Hydrogels for Wound Healing Applications
Abstract
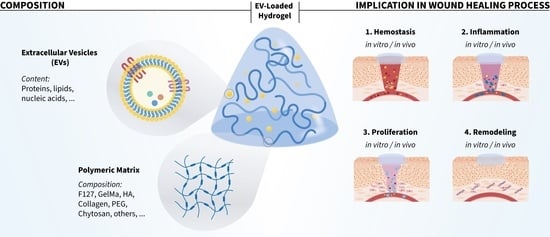
:1. Introduction
2. Suitable EV-Based Hydrogels for Wound Healing Therapies
2.1. Hydrogels
2.2. EVs Source
3. Limitations and Future Perspectives of the Use of EV-Based Hydrogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacques, E.; Suuronen, E.J. The Progression of Regenerative Medicine and Its Impact on Therapy Translation. Clin. Transl. Sci. 2020, 13, 440–450. [Google Scholar] [CrossRef] [Green Version]
- Hoversten, K.P.; Kiemele, L.J.; Stolp, A.M.; Takahashi, P.Y.; Verdoorn, B.P. Prevention, Diagnosis, and Management of Chronic Wounds in Older Adults. Mayo Clin. Proc. 2020, 95, 2021–2034. [Google Scholar] [CrossRef]
- Graves, N.; Phillips, C.J.; Harding, K. A Narrative Review of the Epidemiology and Economics of Chronic Wounds. Br. J. Dermatol. 2022, 187, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Narauskaitė, D.; Vydmantaitė, G.; Rusteikaitė, J.; Sampath, R.; Rudaitytė, A.; Stašytė, G.; Aparicio Calvente, M.I.; Jekabsone, A. Extracellular Vesicles in Skin Wound Healing. Pharmaceuticals 2021, 14, 811. [Google Scholar] [CrossRef] [PubMed]
- Antich-Rosselló, M.; Forteza-Genestra, M.A.; Monjo, M.; Ramis, J.M. Platelet-Derived Extracellular Vesicles for Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 8580. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, W.; Yu, Q.; Qu, W.; Wang, Y.; Li, R. Functional Biomaterials for Treatment of Chronic Wound. Front. Bioeng. Biotechnol. 2020, 8, 516. [Google Scholar] [CrossRef]
- Son, Y.J.; Tse, J.W.; Zhou, Y.; Mao, W.; Yim, E.K.F.; Yoo, H.S. Biomaterials and Controlled Release Strategy for Epithelial Wound Healing. Biomater. Sci. 2019, 7, 4444–4471. [Google Scholar] [CrossRef]
- Lindley, L.E.; Stojadinovic, O.; Pastar, I.; Tomic-Canic, M. Biology and Biomarkers for Wound Healing. Plast. Reconstr. Surg. 2016, 138, 18S–28S. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound Healing: Cellular Mechanisms and Pathological Outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Rumbaut, R.E.; Thiagarajan, P. Platelet-Vessel Wall Interactions in Hemostasis and Thrombosis; Morgan & Claypool Publishers: Kentfield, CA, USA, 2010; Volume 2. [Google Scholar]
- Asada, Y.; Yamashita, A.; Sato, Y.; Hatakeyama, K. Pathophysiology of Atherothrombosis: Mechanisms of Thrombus Formation on Disrupted Atherosclerotic Plaques. Pathol. Int. 2020, 70, 309–322. [Google Scholar] [CrossRef] [Green Version]
- Wilgus, T.A.; Roy, S.; McDaniel, J.C. Neutrophils and Wound Repair: Positive Actions and Negative Reactions. Adv. Wound Care 2013, 2, 379–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, L.C.; Rosas, M.; Jenkins, S.J.; Liao, C.-T.; Scurr, M.J.; Brombacher, F.; Fraser, D.J.; Allen, J.E.; Jones, S.A.; Taylor, P.R. Distinct Bone Marrow-Derived and Tissue-Resident Macrophage Lineages Proliferate at Key Stages during Inflammation. Nat. Commun. 2013, 4, 1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, L.C.; Taylor, P.R. Tissue-Resident Macrophages: Then and Now. Immunology 2015, 144, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Hirschi, K.K. Tissue-Resident Macrophage Development and Function. Front. Cell Dev. Biol. 2020, 8, 617879. [Google Scholar] [CrossRef]
- Rosowski, E.E.; Huttenlocher, A. Neutrophils, Wounds, and Cancer Progression. Dev. Cell 2015, 34, 134–136. [Google Scholar] [CrossRef] [Green Version]
- Bermudez, D.M.; Herdrich, B.J.; Xu, J.; Lind, R.; Beason, D.P.; Mitchell, M.E.; Soslowsky, L.J.; Liechty, K.W. Impaired Biomechanical Properties of Diabetic Skin Implications in Pathogenesis of Diabetic Wound Complications. Am. J. Pathol. 2011, 178, 2215–2223. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, H.N.; Clowes, C.; Banyard, K.L.; Matteuci, P.; Mace, K.A.; Hardman, M.J. Elevated Local Senescence in Diabetic Wound Healing Is Linked to Pathological Repair via CXCR2. J. Investig. Dermatol. 2019, 139, 1171–1181.e6. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Kucheryavenko, O.; Wordsworth, J.; von Zglinicki, T. The Senescent Bystander Effect Is Caused by ROS-Activated NF-ΚB Signalling. Mech. Ageing Dev. 2018, 170, 30–36. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Senescence in Wound Repair: Emerging Strategies to Target Chronic Healing Wounds. Front. Cell Dev. Biol. 2020, 8, 773. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound Senescence: A Functional Link between Diabetes and Ageing? Exp. Dermatol. 2021, 30, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound Healing and Treating Wounds: Chronic Wound Care and Management. J. Am. Acad. Dermatol. 2016, 74, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shi, C. Cellular Senescence Is a Promising Target for Chronic Wounds: A Comprehensive Review. Burn. Trauma 2020, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of Extracellular Vesicles (EV): Exosomes, Microvesicles, Retrovirus-like Vesicles, and Apoptotic Bodies. J. Neurooncol. 2013, 113, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Todorova, D.; Simoncini, S.; Lacroix, R.; Sabatier, F.; Dignat-George, F. Extracellular Vesicles in Angiogenesis. Circ. Res. 2017, 120, 1658–1673. [Google Scholar] [CrossRef]
- Li, M.; Li, S.; Du, C.; Zhang, Y.; Li, Y.; Chu, L.; Han, X.; Galons, H.; Zhang, Y.; Sun, H.; et al. Exosomes from Different Cells: Characteristics, Modifications, and Therapeutic Applications. Eur. J. Med. Chem. 2020, 207, 112784. [Google Scholar] [CrossRef]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Fang, F.; Sun, M.; Zhang, Y.; Hu, M.; Zhang, J. Extracellular Vesicles as Bioactive Nanotherapeutics: An Emerging Paradigm for Regenerative Medicine. Theranostics 2022, 12, 4879–4903. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, Y.; Liu, S.; Ge, S. Biomaterials Constructed for MSC-Derived Extracellular Vesicle Loading and Delivery-a Promising Method for Tissue Regeneration. Front. Cell Dev. Biol. 2022, 10, 898394. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Qin, D.; Sun, M.; Wang, T.; Chen, X. Research Status of Self-Healing Hydrogel for Wound Management: A Review. Int. J. Biol. Macromol. 2020, 164, 2108–2123. [Google Scholar] [CrossRef]
- Yao, H.; Yuan, X.; Wu, Z.; Park, S.; Zhang, W.; Chong, H.; Lin, L.; Piao, Y. Fabrication and Performance Evaluation of Gelatin/Sodium Alginate Hydrogel-Based Macrophage and MSC Cell-Encapsulated Paracrine System with Potential Application in Wound Healing. Int. J. Mol. Sci. 2023, 24, 1240. [Google Scholar] [CrossRef]
- Monteiro, N.; Martins, M.; Martins, A.; Fonseca, N.A.; Moreira, J.N.; Reis, R.L.; Neves, N.M. Antibacterial Activity of Chitosan Nanofiber Meshes with Liposomes Immobilized Releasing Gentamicin. Acta Biomater. 2015, 18, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Wu, M.; Lin, L.; Wu, Z.; Bae, M.; Park, S.; Wang, S.; Zhang, W.; Gao, J.; Wang, D.; et al. Design Strategies for Adhesive Hydrogels with Natural Antibacterial Agents as Wound Dressings: Status and Trends. Mater. Today Bio 2022, 16, 100429. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Wu, Q.; Wang, Y.; Zhang, D.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. A Biodegradable Hydrogel System Containing Curcumin Encapsulated in Micelles for Cutaneous Wound Healing. Biomaterials 2013, 34, 6377–6387. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Paras, C.B.; Weng, H.; Punnakitikashem, P.; Su, L.-C.; Vu, K.; Tang, L.; Yang, J.; Nguyen, K.T. Dual Growth Factor Releasing Multi-Functional Nanofibers for Wound Healing. Acta Biomater. 2013, 9, 9351–9359. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhou, S.; Gao, Y.; Zhai, Y. Electrospun Nanofibers as a Wound Dressing for Treating Diabetic Foot Ulcer. Asian J. Pharm. Sci. 2019, 14, 130–143. [Google Scholar] [CrossRef]
- Charernsriwilaiwat, N.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Lysozyme-Loaded, Electrospun Chitosan-Based Nanofiber Mats for Wound Healing. Int. J. Pharm. 2012, 427, 379–384. [Google Scholar] [CrossRef]
- Kim, H.S.; Yoo, H.S. In Vitro and in Vivo Epidermal Growth Factor Gene Therapy for Diabetic Ulcers with Electrospun Fibrous Meshes. Acta Biomater. 2013, 9, 7371–7380. [Google Scholar] [CrossRef]
- Ifuku, S.; Hori, T.; Izawa, H.; Morimoto, M.; Saimoto, H. Preparation of Zwitterionically Charged Nanocrystals by Surface TEMPO-Mediated Oxidation and Partial Deacetylation of α-Chitin. Carbohydr. Polym. 2015, 122, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Araújo, J.V.; Reis, R.L.; Neves, N.M. Electrospun Nanostructured Scaffolds for Tissue Engineering Applications. Nanomedicine 2007, 2, 929–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Liu, B.; Carlson, M.A.; Gombart, A.F.; Reilly, D.A.; Xie, J. Recent Advances in Electrospun Nanofibers for Wound Healing. Nanomedicine 2017, 12, 1335–1352. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-L.; Fu, R.-H.; Liao, S.-F.; Liu, S.-P.; Lin, S.-Z.; Wang, Y.-C. A PEG-Based Hydrogel for Effective Wound Care Management. Cell Transplant. 2018, 27, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, D.P.; Aguilar, L.E.; Park, C.H.; Kim, C.S. A Review on Properties of Natural and Synthetic Based Electrospun Fibrous Materials for Bone Tissue Engineering. Membranes 2018, 8, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parlato, M.; Reichert, S.; Barney, N.; Murphy, W.L. Poly(Ethylene Glycol) Hydrogels with Adaptable Mechanical and Degradation Properties for Use in Biomedical Applications. Macromol. Biosci. 2014, 14, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Babavalian, H.; Latifi, A.M.; Shokrgozar, M.A.; Bonakdar, S.; Shakeri, F.; Tebyanian, H. Healing Effects of Synthetic versus Commercial Alginate Hydrogel Dressings on Wounds. Trauma Mon. 2017, 22, e64270. [Google Scholar] [CrossRef]
- Aswathy, S.H.; Narendrakumar, U.; Manjubala, I. Commercial Hydrogels for Biomedical Applications. Heliyon 2020, 6, 719. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Fuhrmann, G. Extracellular Vesicles—A Versatile Biomaterial. Adv. Healthc. Mater. 2022, 11, 2200192. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, G.; Huang, H.; Wang, K.; Wang, H.; Lang, M.; Gao, H.; Zhao, S. Sequential Release of Small Extracellular Vesicles from Bilayered Thiolated Alginate/Polyethylene Glycol Diacrylate Hydrogels for Scarless Wound Healing. ACS Nano 2021, 15, 6352–6368. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Liu, K.; Jiang, T.; Li, S.; Chen, J.; Wu, Z.; Li, W.; Tan, R.; Wei, W.; Yang, X.; et al. GelMA/PEGDA Microneedles Patch Loaded with HUVECs-Derived Exosomes and Tazarotene Promote Diabetic Wound Healing. J. Nanobiotechnology 2022, 20, 147. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Qi, Y.; Liu, X.; Shi, Y.; Li, H.; Zhao, L. A Multifunctional Antibacterial and Self-Healing Hydrogel Laden with Bone Marrow Mesenchymal Stem Cell-Derived Exosomes for Accelerating Diabetic Wound Healing. Biomater. Adv. 2022, 133, 112613. [Google Scholar] [CrossRef]
- Su, Y.; Sharma, N.S.; John, J.V.; Ganguli-Indra, G.; Indra, A.K.; Gombart, A.F.; Xie, J. Engineered Exosomes Containing Cathelicidin/LL-37 Exhibit Multiple Biological Functions. Adv. Healthc. Mater. 2022, 11, e2200849. [Google Scholar] [CrossRef]
- Xie, Y.; Guan, Q.; Guo, J.; Chen, Y.; Yin, Y.; Han, X. Hydrogels for Exosome Delivery in Biomedical Applications. Gels 2022, 8, 328. [Google Scholar] [CrossRef]
- Chabria, Y.; Duffy, G.P.; Lowery, A.J.; Dwyer, R.M. Hydrogels: 3D Drug Delivery Systems for Nanoparticles and Extracellular Vesicles. Biomedicines 2021, 9, 1694. [Google Scholar] [CrossRef]
- Wang, C.-H.; Cherng, J.-H.; Liu, C.-C.; Fang, T.-J.; Hong, Z.-J.; Chang, S.-J.; Fan, G.-Y.; Hsu, S.-D. Procoagulant and Antimicrobial Effects of Chitosan in Wound Healing. Int. J. Mol. Sci. 2021, 22, 7067. [Google Scholar] [CrossRef]
- Frazier, T.; Alarcon, A.; Wu, X.; Mohiuddin, O.A.; Motherwell, J.M.; Carlsson, A.H.; Christy, R.J.; Edwards, J.V.; Mackin, R.T.; Prevost, N.; et al. Clinical Translational Potential in Skin Wound Regeneration for Adipose-Derived, Blood-Derived, and Cellulose Materials: Cells, Exosomes, and Hydrogels. Biomolecules 2020, 10, 1373. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Bai, Y.; Zhou, J.; Li, L.; Na, J.; Fan, Y.; Guo, X.; Liu, H. A Moisturizing Chitosan-Silk Fibroin Dressing with Silver Nanoparticles-Adsorbed Exosomes for Repairing Infected Wounds. J. Mater. Chem. B 2020, 8, 7197–7212. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Wang, L.; Guan, J.; Tang, C.; He, N.; Zhang, W.; Fu, S. Wound Healing Effects of a Curcuma Zedoaria Polysaccharide with Platelet-Rich Plasma Exosomes Assembled on Chitosan/Silk Hydrogel Sponge in a Diabetic Rat Model. Int. J. Biol. Macromol. 2018, 117, 102–107. [Google Scholar] [CrossRef]
- Nooshabadi, V.T.; Khanmohamadi, M.; Valipour, E.; Mahdipour, S.; Salati, A.; Malekshahi, Z.V.; Shafei, S.; Amini, E.; Farzamfar, S.; Ai, J. Impact of Exosome-Loaded Chitosan Hydrogel in Wound Repair and Layered Dermal Reconstitution in Mice Animal Model. J. Biomed. Mater. Res. A 2020, 108, 2138–2149. [Google Scholar] [CrossRef]
- Abolgheit, S.; Abdelkader, S.; Aboushelib, M.; Omar, E.; Mehanna, R. Bone Marrow-Derived Mesenchymal Stem Cells and Extracellular Vesicles Enriched Collagen Chitosan Scaffold in Skin Wound Healing (a Rat Model). J. Biomater. Appl. 2021, 36, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Guo, S.; Li, M.; Ke, Q.; Guo, Y.; Zhang, C. Chitosan Wound Dressings Incorporating Exosomes Derived from MicroRNA-126-Overexpressing Synovium Mesenchymal Stem Cells Provide Sustained Release of Exosomes and Heal Full-Thickness Skin Defects in a Diabetic Rat Model. Stem Cells Transl. Med. 2017, 6, 736. [Google Scholar] [CrossRef]
- Tang, Q.; Lu, B.; He, J.; Chen, X.; Fu, Q.; Han, H.; Luo, C.; Yin, H.; Qin, Z.; Lyu, D.; et al. Exosomes-Loaded Thermosensitive Hydrogels for Corneal Epithelium and Stroma Regeneration. Biomaterials 2022, 280, 121320. [Google Scholar] [CrossRef]
- Antich-Rosselló, M.; Munar-Bestard, M.; Forteza-Genestra, M.A.; Calvo, J.; Gayà, A.; Monjo, M.; Ramis, J.M. Evaluation of Platelet-Derived Extracellular Vesicles in Gingival Fibroblasts and Keratinocytes for Periodontal Applications. Int. J. Mol. Sci. 2022, 23, 7668. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Z.; Li, Y.; Wang, Y.; Li, Q.; Han, D. GelMA Combined with Sustained Release of HUVECs Derived Exosomes for Promoting Cutaneous Wound Healing and Facilitating Skin Regeneration. J. Mol. Histol. 2020, 51, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, Z.; Wei, Q.; Ma, K.; Hu, W.; Huang, Q.; Su, J.; Li, H.; Zhang, C.; Fu, X. VH298-Loaded Extracellular Vesicles Released from Gelatin Methacryloyl Hydrogel Facilitate Diabetic Wound Healing by HIF-1α-Mediated Enhancement of Angiogenesis. Acta Biomater. 2022, 147, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, O.J.; Alvarez, S.; Contreras-Kallens, P.; Barrera, N.P.; Aguayo, S.; Schuh, C.M.A.P. Type I Collagen Hydrogels as a Delivery Matrix for Royal Jelly Derived Extracellular Vesicles. Drug Deliv. 2020, 27, 1308–1318. [Google Scholar] [CrossRef]
- Hao, D.; Lu, L.; Song, H.; Duan, Y.; Chen, J.; Carney, R.; Li, J.J.; Zhou, P.; Nolta, J.; Lam, K.S.; et al. Engineered Extracellular Vesicles with High Collagen-Binding Affinity Present Superior in Situ Retention and Therapeutic Efficacy in Tissue Repair. Theranostics 2022, 12, 6021–6037. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Tang, L.; Xiao, H.; Yang, Z.; Wang, S. Preparation of Recombinant Human Collagen III Protein Hydrogels with Sustained Release of Extracellular Vesicles for Skin Wound Healing. Int. J. Mol. Sci. 2022, 23, 6289. [Google Scholar] [CrossRef]
- Teng, L.; Maqsood, M.; Zhu, M.; Zhou, Y.; Kang, M.; Zhou, J.; Chen, J. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Accelerate Diabetic Wound Healing via Promoting M2 Macrophage Polarization, Angiogenesis, and Collagen Deposition. Int. J. Mol. Sci. 2022, 23, 10421. [Google Scholar] [CrossRef]
- Wang, C.; Liang, C.; Wang, R.; Yao, X.; Guo, P.; Yuan, W.; Liu, Y.; Song, Y.; Li, Z.; Xie, X. The Fabrication of a Highly Efficient Self-Healing Hydrogel from Natural Biopolymers Loaded with Exosomes for the Synergistic Promotion of Severe Wound Healing. Biomater. Sci. 2019, 8, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Trenkenschuh, E.; Richter, M.; Heinrich, E.; Koch, M.; Fuhrmann, G.; Friess, W. Enhancing the Stabilization Potential of Lyophilization for Extracellular Vesicles. Adv. Healthc. Mater. 2021, 11, e2100538. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Z.; Pan, D.; Li, H.; Shen, J. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. Int. J. Nanomed. 2020, 15, 5911–5926. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Hu, Y.; Yang, P.; Xie, X.; Fang, B. Extracellular Vesicle-Loaded Hydrogels for Tissue Repair and Regeneration. Mater. Today Bio 2023, 18, 100522. [Google Scholar] [CrossRef]
- Safari, B.; Aghazadeh, M.; Davaran, S.; Roshangar, L. Exosome-Loaded Hydrogels: A New Cell-Free Therapeutic Approach for Skin Regeneration. Eur. J. Pharm. Biopharm. 2022, 171, 50–59. [Google Scholar] [CrossRef] [PubMed]
- de Paula, M.M.M.; Bassous, N.J.; Afewerki, S.; Harb, S.V.; Ghannadian, P.; Marciano, F.R.; Viana, B.C.; Tim, C.R.; Webster, T.J.; Lobo, A.O. Understanding the Impact of Crosslinked PCL/PEG/GelMA Electrospun Nanofibers on Bactericidal Activity. PLoS ONE 2018, 13, e0209386. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Dong, Y.; Xu, P.; Pan, Q.; Jia, K.; Jin, P.; Zhou, M.; Xu, Y.; Guo, R.; Cheng, B. A Composite Hydrogel Containing Resveratrol-Laden Nanoparticles and Platelet-Derived Extracellular Vesicles Promotes Wound Healing in Diabetic Mice. Acta Biomater. 2022, 154, 212–230. [Google Scholar] [CrossRef]
- Verweij, F.J.; Revenu, C.; Arras, G.; Dingli, F.; Loew, D.; Pegtel, D.M.; Follain, G.; Allio, G.; Goetz, J.G.; Zimmermann, P.; et al. Live Tracking of Inter-Organ Communication by Endogenous Exosomes In Vivo. Dev. Cell 2019, 48, 573–589.e4. [Google Scholar] [CrossRef] [Green Version]
- Shiekh, P.A.; Singh, A.; Kumar, A. Exosome Laden Oxygen Releasing Antioxidant and Antibacterial Cryogel Wound Dressing OxOBand Alleviate Diabetic and Infectious Wound Healing. Biomaterials 2020, 249, 120020. [Google Scholar] [CrossRef]
- Shafei, S.; Khanmohammadi, M.; Heidari, R.; Ghanbari, H.; Taghdiri Nooshabadi, V.; Farzamfar, S.; Akbariqomi, M.; Sanikhani, N.S.; Absalan, M.; Tavoosidana, G. Exosome Loaded Alginate Hydrogel Promotes Tissue Regeneration in Full-Thickness Skin Wounds: An in Vivo Study. J. Biomed. Mater. Res. A 2020, 108, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Chen, L.; Liu, P.; Yu, T.; Lin, C.; Yan, C.; Hu, Y.; Zhou, W.; Sun, Y.; Panayi, A.C.; et al. All-in-One: Multifunctional Hydrogel Accelerates Oxidative Diabetic Wound Healing through Timed-Release of Exosome and Fibroblast Growth Factor. Small 2022, 18, e2104229. [Google Scholar] [CrossRef]
- Kwak, G.; Cheng, J.; Kim, H.; Song, S.; Lee, S.J.; Yang, Y.; Jeong, J.H.; Lee, J.E.; Messersmith, P.B.; Kim, S.H. Sustained Exosome-Guided Macrophage Polarization Using Hydrolytically Degradable PEG Hydrogels for Cutaneous Wound Healing: Identification of Key Proteins and MiRNAs, and Sustained Release Formulation. Small 2022, 18, e2200060. [Google Scholar] [CrossRef]
- Görgens, A.; Corso, G.; Hagey, D.W.; Jawad Wiklander, R.; Gustafsson, M.O.; Felldin, U.; Lee, Y.; Bostancioglu, R.B.; Sork, H.; Liang, X.; et al. Identification of Storage Conditions Stabilizing Extracellular Vesicles Preparations. J. Extracell. Vesicles 2022, 11, e12238. [Google Scholar] [CrossRef] [PubMed]
- Forteza-Genestra, M.A.; Antich-Rosselló, M.; Calvo, J.; Gayà, A.; Monjo, M.; Ramis, J.M. Purity Determines the Effect of Extracellular Vesicles Derived from Mesenchymal Stromal Cells. Cells 2020, 9, 422. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Gong, S.; Yao, W.; Yang, Z.; Wang, R.; Yu, Z.; Wei, M. Exosome Loaded Genipin Crosslinked Hydrogel Facilitates Full Thickness Cutaneous Wound Healing in Rat Animal Model. Drug Deliv. 2021, 28, 884–893. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Shi, L.; Li, B.; Li, J.; Wei, Z.; Lv, H.; Wu, L.; Zhang, H.; Yang, B.; et al. Magnetic Targeting Enhances the Cutaneous Wound Healing Effects of Human Mesenchymal Stem Cell-Derived Iron Oxide Exosomes. J. Nanobiotechnology 2020, 18, 113. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Zhao, Y.; Wu, M.; Mao, S.; Cong, P.; Zou, R.; Hou, M.; Jin, H.; Bao, Y. Application of Adipose Mesenchymal Stem Cell-Derived Exosomes-Loaded β-Chitin Nanofiber Hydrogel for Wound Healing. Folia Histochem. Et Cytobiol. 2022, 60, 167–178. [Google Scholar] [CrossRef]
- Guo, S.-C.; Tao, S.-C.; Yin, W.-J.; Qi, X.; Yuan, T.; Zhang, C.-Q. Exosomes Derived from Platelet-Rich Plasma Promote the Re-Epithelization of Chronic Cutaneous Wounds via Activation of YAP in a Diabetic Rat Model. Theranostics 2017, 7, 81–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Wang, C.; Chen, M.; Xi, Y.; Cheng, W.; Mao, C.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; et al. Efficient Angiogenesis-Based Diabetic Wound Healing/Skin Reconstruction through Bioactive Antibacterial Adhesive Ultraviolet Shielding Nanodressing with Exosome Release. ACS Nano 2019, 13, 10279–10293. [Google Scholar] [CrossRef]
- Ferroni, L.; Gardin, C.; D’Amora, U.; Calzà, L.; Ronca, A.; Tremoli, E.; Ambrosio, L.; Zavan, B. Exosomes of Mesenchymal Stem Cells Delivered from Methacrylated Hyaluronic Acid Patch Improve the Regenerative Properties of Endothelial and Dermal Cells. Biomater. Adv. 2022, 139, 213000. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, X.-L.; Lu, S.-T.; Zhang, N.-Y.; Zhang, H.-J.; Zhang, J.; Zhang, J. Human Adipose-Derived Mesenchymal Stem Cells-Derived Exosomes Encapsulated in Pluronic F127 Hydrogel Promote Wound Healing and Regeneration. Stem Cell Res. Ther. 2022, 13, 407. [Google Scholar] [CrossRef]
- Li, J.; Yan, S.; Han, W.; Dong, Z.; Li, J.; Wu, Q.; Fu, X. Phospholipid-Grafted PLLA Electrospun Micro/Nanofibers Immobilized with Small Extracellular Vesicles from Rat Adipose Mesenchymal Stem Cells Promote Wound Healing in Diabetic Rats. Regen. Biomater. 2022, 9, rbac071. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Hu, H.; Wu, J.; Li, X.; Ma, X.; Zhao, Z.; Liu, Z.; Wu, C.; Zhao, B.; Wang, Y.; et al. Functional Extracellular Matrix Hydrogel Modified with MSC-Derived Small Extracellular Vesicles for Chronic Wound Healing. Cell Prolif. 2022, 55, e13196. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Jiang, H.; Qian, M.; Ji, G.; Wei, Y.; He, J.; Tian, H.; Zhao, Q. MSC-Derived SEV-Loaded Hyaluronan Hydrogel Promotes Scarless Skin Healing by Immunomodulation in a Large Skin Wound Model. Biomed. Mater. 2022, 17, 034104. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wu, F.; Wang, X.; Song, Q.; Ye, Z.; Mohammadniaei, M.; Zhang, M.; Chu, X.; Xi, S.; Zhou, N.; et al. An Optimally Designed Engineering Exosome-Reductive COF Integrated Nanoagent for Synergistically Enhanced Diabetic Fester Wound Healing. Small 2022, 18, 2200895. [Google Scholar] [CrossRef]


| EVs Source | Biomaterial | Aim of Clinical Use | Cell and Molecular Mechanism of Wound Closure | Reference | |
|---|---|---|---|---|---|
| Adipose | MSC | FHE Hydrogel | Diabetic WH | ↑ Angiogenesis, collagen deposition, re-epithelization | [51] |
| Methacrylate HA | WH | ↑ Cell proliferation, migration, angiogenesis, and WH-related marker expression in fibroblast and endothelial cells | [92] | ||
| PUAO and PUAO-CPO cryogel | Diabetic and infectious WH | ↑ Angiogenesis, re-epithelization, fibroblasts, and keratinocytes migration | [81] | ||
| Alginate hydrogel | WH | ↑ Angiogenesis, re-epithelization, collagen deposition, and remodeling | [82] | ||
| Pluronic F127 hydrogel | Skin WH | ↑ Skin WH, re-epithelialization, expression of Ki67, α-SMA, CD31, collagen synthesis (Collagen I, Collagen III), and skin barrier proteins (KRT1, AQP3). ↓ Inflammation (IL-6, TNF-α, CD68, CD206) | [93] | ||
| β-ChNF hydrogel | Skin WH | Metabolic, tight junction, NF-κB signaling pathways, CFD, downstream Aldolase A, and Actn2 proteins | [89] | ||
| Polysaccharide based dressing | Diabetic WH and angiogenesis | ↑ Angiogenesis, re-epithelization, collagen deposition, and remodeling | [91] | ||
| Phospholipid-grafted PLLA electrospun micro/nanofibers immobilized | Diabetic WH | ↑ Fibroblast proliferation, migration, and gene expression (Collagen I and III, TGF-β, α-SMA, HIF-1α). ↑ Keratinocyte proliferation. ↑ Expression of anti-inflammatory genes (Arg1, CD206, IL-10). ↓ Expression of pro-inflammatory genes (IL-1β, TNF-α). ↑ Cell proliferation, collagen deposition, and angiogenesis | [94] | ||
| Umbilical Cord | Recombinant Human Collagen III Protein Hydrogels | Diabetic WH | ↓ Inflammatory response. ↑ Cell proliferation and angiogenesis | [71] | |
| Peptides based hydrogel | Diabetic WH | ↑ Stimulating angiogenesis capacity | [95] | ||
| Iron Oxide exosomes | Skin WH | ↑ Endothelial cell proliferation, migration, and angiogenic tubule formation. ↓ Scar formation. ↑ CK19, PCNA, and collagen expression | [87] | ||
| Pluronic F127 Hydrogel | Diabetic WH | VEGF and TGFβ | [75] | ||
| Genipin crosslinked hydrogel | Skin WH | ↑ Wound closure, re-epithelialization, collagen deposition, and several skin appendages | [87] | ||
| Chitosan-SF/SA/Ag dressing | Skin WH | Antimicrobial activity. ↑ WH, retaining moisture, maintaining electrolyte balance, fibroblast proliferation, collagen deposition, angiogenesis, and nerve repair | [60] | ||
| Placental | Hyaluroran hydrogel | Scarless WH | ↓ Scar tissue formation. Macrophages induction to an anti-inflammatory and anti-fibrotic (M2c) phenotype | [96] | |
| Chitosan—PEG Hydrogel | Skin WH | ↑ Induced proliferation and vascular formation | [73] | ||
| Collagen biomaterial | Skin WH | ↓ Inflammatory responses. ↑ Muscle regeneration and vascularization | [70] | ||
| Synovium | Chitosan wound dressing | Diabetic WH | ↑ Collagen deposition, angiogenesis, and re-epithelization | [64] | |
| iPSC | Chitosan-based hydrogels | Corneal epithelium regeneration | (miR-432-5p)-mediated action | [65] | |
| Bone marrow | CEC-DCMC hydrogel | Diabetic WH | ↑ Angiogenesis, WH, and M1-type to M2-type transition of macrophages. ↓ Inflammatory effects | [54] | |
| Bilayered Thiolated Alginate/PEG Diacrylate Hydrogels | Scarless WH | (miR-29-b-3p)-mediated action. ↑ Angiogenesis and re-epithelization | [52] | ||
| Collagen chitosan scaffold | WH | ↑ Macrophages count, collagen deposition, and alignment | [63] | ||
| TNF-α and hypoxia treated | COF Integrated Nanoagent | Diabetic WH | ↑ Anti-inflammatory M2 macrophage polarization, stabilization of HIF-1α, and angiogenesis. ↓ Oxidative injury, tissue inflammation, and bacterial infection | [97] | |
| hEnSC | Chitosan-glycerol hydrogel | Skin WH | ↑ Wound closure ability and re-epithelialization | [62] | |
| ESC | GelMA hydrogel | Diabetic WH | ↑ Angiogenesis by stabilizing HIF1α | [68] | |
| HUVEC | GelMA/PEGDA | Diabetic WH | ↑ Cell migration, angiogenesis, and exosomes/tazarotene release in the deep skin layer | [53] | |
| GelMA hydrogel | Skin WH | ↑ Re-epithelialization, collagen maturity, and angiogenesis | [67] | ||
| M2-Macrophages | HA@MnO2 /FGF-2 hydrogel | Diabetic WH | ↑ Angiogenesis, ROS depletion, collagen deposition, and remodelation | [83] | |
| Hydrolytically degradable PEG hydrogels | Cutaneous WH | The regulated local polarization state of Mφs and local transition from M1-Mφs to M2-Mφs within the lesion. ↑ Wound closure and increased healing quality | [84] | ||
| Monocytic cells | Electrospun nanofiber matrices | Wound infection | Bactericidal effect. ↑ HUVEC tube formation, skin cell proliferation, and migration | [55] | |
| Platelets/PRP | HA-based hydrogels | Gingival WH | Preserved activity and functionality of platelet-derived EVs | [66] | |
| GelMA/SFMA/MSN-RES hidrogel | Diabetic WH | ↓ Macrophage iNOS expression. ↓ Expression of TNF-α. ↑ Tube formation by hUVEC in vitro. ↑ Angiogenesis, expression of TGF-β1, Arg-1, extracellular purinergic signaling pathway-related CD73, and A2A-R | [79] | ||
| Curcuma polysaccharide-based chitosan/silk hydrogel sponge | Diabetic WH | ↑ Collagen deposition and angiogenesis | [61] | ||
| Apis mellifera royal jelly | Collagen Type I Hydrogel | Skin WH | ↑ Fibroblast contractile capacity and migration. ↓ Staphylococcus aureus ATCC 29213 biofilm formation | [69] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amengual-Tugores, A.M.; Ráez-Meseguer, C.; Forteza-Genestra, M.A.; Monjo, M.; Ramis, J.M. Extracellular Vesicle-Based Hydrogels for Wound Healing Applications. Int. J. Mol. Sci. 2023, 24, 4104. https://doi.org/10.3390/ijms24044104
Amengual-Tugores AM, Ráez-Meseguer C, Forteza-Genestra MA, Monjo M, Ramis JM. Extracellular Vesicle-Based Hydrogels for Wound Healing Applications. International Journal of Molecular Sciences. 2023; 24(4):4104. https://doi.org/10.3390/ijms24044104
Chicago/Turabian StyleAmengual-Tugores, Andreu Miquel, Carmen Ráez-Meseguer, Maria Antònia Forteza-Genestra, Marta Monjo, and Joana M. Ramis. 2023. "Extracellular Vesicle-Based Hydrogels for Wound Healing Applications" International Journal of Molecular Sciences 24, no. 4: 4104. https://doi.org/10.3390/ijms24044104
APA StyleAmengual-Tugores, A. M., Ráez-Meseguer, C., Forteza-Genestra, M. A., Monjo, M., & Ramis, J. M. (2023). Extracellular Vesicle-Based Hydrogels for Wound Healing Applications. International Journal of Molecular Sciences, 24(4), 4104. https://doi.org/10.3390/ijms24044104