Spermidine-Eugenol Supplement Preserved Inflammation-Challenged Intestinal Cells by Stimulating Autophagy
Abstract
:1. Introduction
2. Results
2.1. Spermidine Composition of the Supplement
2.2. Effects Spermidine and Eugenol Treatments on Autophagy LC3-II Marker Detection and Tolerability in Caco-2 and NCM460 Cell Lines
2.3. Effects of Spermidine and Eugenol Treatments on Autophagy LC3-II Marker Turnover in NCM460 Cell Lines in the Presence and Absence of Liposaccharide
2.4. Effect of the Spermidine and Eugenol Combination and Supplement Pretreatments on the Expression of Autophagy Microarray Expression in NCM460 Cells Treated with Lipopolysaccharide
2.5. Effect of Spermidine and Eugenol Pretreatments on Inflammatory Parameters in NCM460 Cells Treated with Lipopolysaccharide
2.6. Effect of Spermidine plus Eugenol and Supplement Pretreatments in Reconstituted Caco-2 Intestinal Equivalents Exposed to Liposaccharide
2.7. Effect of the Supplement Pretreatments on the Expression of LC3-II Marker Detection and Midkine Expression in NCM460 Cells Treated with Lipopolysaccharide and Compound C
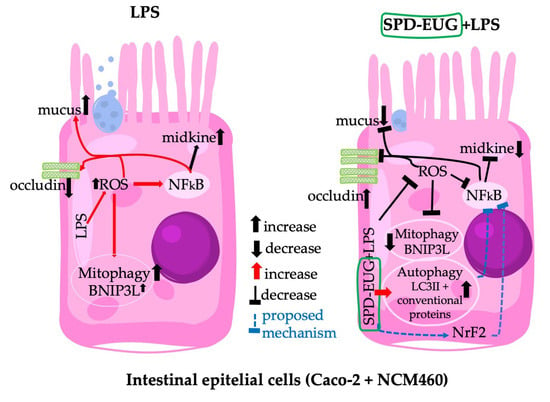
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Polyamine Extraction and Measurement
4.3. Cell Model Systems and Growth Maintenance Conditions
4.4. Experimental Monoculture Conditions to Investigate Spermidine and Eugenol Treatments in the Absence or Presence of the Inflammation Elicitor Lipopolysaccharide
4.5. Cell Proliferation (MTT) Assay
4.6. Immunocytochemistry for Autophagy LC3-II Marker Detection
4.7. Immunofluorescence and Immunocytochemistry for Autophagy LC3-II and P62 Expression and Turnover with Hydroxychloroquine
4.8. ROS and Midkine Detection
4.9. Detection of Autophagy Protein Activation
4.10. Autophagy LC3-II Marker, Occludin, and Mucus Detection in a Reconstituted Intestinal Cell Model with Caco-2, U937, and L929 Cells
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netea-Maier, R.T.; Plantinga, T.S.; van de Veerdonk, F.L.; Smit, J.W.; Netea, M.G. Modulation of inflammation by autophagy: Consequences for human disease. Autophagy 2016, 12, 245–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naveja, J.J.; Madariaga-Mazón, A.; Flores-Murrieta, F.J.; Granados-Montiel, J.; Maradiaga-Ceceña, M.A.; Alaniz, V.D.; Maldonado-Rodríguez, M.R.; García-Morales, J.; Senosiain-Peláez, J.P.; Martínez-Mayorga, K. Union is strength: Antiviral and anti-inflammatory drugs for COVID-19. Drug Discov. Today 2021, 26, 29–239. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.; Leão, A.; Erustes, A.G.; Morais, I.; Vrechi, T.; Zamarioli, L.; Pereira, C.; Marchioro, L.O.; Sperandio, L.P.; Lins, Í.; et al. Pharmacological Modulators of Autophagy as a Potential Strategy for the Treatment of COVID-19. Int. J. Mol. Sci. 2021, 22, 4067. [Google Scholar] [CrossRef]
- Seiler, K.; Tschan, M.P.P. Autophagy and coronavirus infection—A Trojan horse or Achilles’ heel? Swiss Med. Wkly. 2021, 151, w20468. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Lu, K.; Mao, B.; Liu, S.; Trilling, M.; Huang, A.; Lu, M.; Lin, Y. The interplay between emerging human coronavirus infections and autophagy. Emerg. Microbes Infect. 2021, 10, 196–205. [Google Scholar] [CrossRef]
- Alsaleh, G.; Panse, I.; Swadling, L.; Zhang, H.; Richter, F.C.; Meyer, A.; Lord, J.; Barnes, E.; Klenerman, P.; Green, C.; et al. Autophagy in T cells from aged donors is maintained by spermidine and correlates with function and vaccine responses. eLife 2020, 9, e57950. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Petroni, G.; Amaravadi, R.K.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cadwell, K.; Cecconi, F.; Choi, A.; et al. Autophagy in major human diseases. EMBO J. 2021, 40, e108863. [Google Scholar] [CrossRef]
- Zinecker, H.; Simon, A.K. Autophagy takes it all-autophagy inducers target immune aging. Dis. Models Mech. 2022, 15, dmm049345. [Google Scholar] [CrossRef]
- Zhou, M.; Xu, W.; Wang, J.; Yan, J.; Shi, Y.; Zhang, C.; Ge, W.; Wu, J.; Du, P.; Chen, Y. Boosting mTOR-dependent autophagy via upstream TLR4-MyD88-MAPK signalling and downstream NF-κB pathway quenches intestinal inflammation and oxidative stress injury. EBioMedicine 2018, 35, 345–360. [Google Scholar] [CrossRef] [Green Version]
- Saitoh, T.; Fujita, N.; Jang, M.H.; Uematsu, S.; Yang, B.G.; Satoh, T.; Omori, H.; Noda, T.; Yamamoto, N.; Komatsu, M.; et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 2008, 456, 264–268. [Google Scholar] [CrossRef]
- Haq, S.; Grondin, J.; Banskota, S.; Khan, W. Autophagy: Roles in intestinal mucosal homeostasis and inflammation. J. Biomed. Sci. 2019, 26, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, L.; Pan, Q.; Yang, N. Autophagy and inflammation regulation in acute kidney Injury. Front. Physiol. 2020, 11, 576463. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Zhang, D.; Geng, H.; Sun, F.; Lei, M. Salidroside protects endothelial cells against LPS-induced inflammatory injury by inhibiting NLRP3 and enhancing autophagy. BMC Complement. Med. Ther. 2021, 21, 146. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Baek, A.; Kim, D.E. Autophagy down-regulates NLRP3-dependent inflammatory response of intestinal epithelial cells under nutrient deprivation. BMB Rep. 2021, 4, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, Y.; Nishiumi, S.; Masuda, A.; Inoue, J.; Nguyen, N.M.; Irino, Y.; Komatsu, M.; Tanaka, K.; Kutsumi, H.; Azuma, T.; et al. Autophagy in the intestinal epithelium reduces endotoxin-induced inflammatory responses by inhibiting NF-kappaB activation. Arch. Biochem. Biophys. 2011, 506, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Park, H.Y. Anti-inflammatory effects of spermidine in lipopolysaccharide-stimulated BV2 microglial cells. J. Biomed. Sci. 2012, 19, 31. [Google Scholar] [CrossRef] [Green Version]
- Ni, Y.Q.; Liu, Y.S. New insights into the roles and mechanisms of spermidine in aging and age-related diseases. Aging Dis. 2021, 12, 1948–1963. [Google Scholar] [CrossRef]
- Schwarz, C.; Stekovic, S.; Wirth, M.; Benson, G.; Royer, P.; Sigrist, S.J.; Pieber, T.; Dammbruec, C.; Magnes, C.; Eisenberg, T.; et al. Safety and tolerability of spermidine supplementation in mice and older adults with subjective cognitive decline. Aging 2018, 10, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Allison, L. Spermidine: The Science behind Longevity Supplements. Available online: https://longevity.technology/lifestyle/spermidine-the-science-behind-spermidine-supplements/ (accessed on 31 January 2023).
- Truzzi, F.; Whittaker, A.; D’Amen, E.; Tibaldi, C.; Abate, A.; Valerii, M.C.; Spisni, E.; Dinelli, G. Wheat germ spermidine and clove eugenol in combination stimulate autophagy in vitro showing potential in supporting the immune system against viral infections. Molecules 2022, 27, 3425. [Google Scholar] [CrossRef]
- Spisni, E.; Petrocelli, G.; Imbesi, V.; Spigarelli, R.; Azzinnari, D.; Donati Sarti, M.; Campieri, M.; Valerii, M.C. Antioxidant, anti-inflammatory, and microbial-modulating activities of essential oils: Implications in colonic pathophysiology. Int. J. Mol. Sci. 2020, 21, 4152. [Google Scholar] [CrossRef] [PubMed]
- Ulanowska, M.; Olas, B. Biological properties and prospects for the application of eugenol—A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yan, J.Y.; Wang, Y.X.; Ling, Y.N.; Song, X.D.; Wang, S.Y.; Liu, H.Q.; Liu, Q.C.; Zhang, Y.; Yang, P.Z.; et al. Spermidine-enhanced autophagic flux improves cardiac dysfunction following myocardial infarction by targeting the AMPK/mTOR signalling pathway. Br. J. Pharmacol. 2019, 176, 3126–3142. [Google Scholar] [CrossRef]
- Liu, R.; Li, X.; Ma, H.; Yang, Q.; Shang, Q.; Song, L.; Zheng, Z.; Zhang, S.; Pan, Y.; Huang, P.; et al. Spermidine endows macrophages anti-inflammatory properties by inducing mitochondrial superoxide-dependent AMPK activation, Hif-1α upregulation and autophagy. Free Radic. Biol. Med. 2020, 161, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Carriche, G.M.; Almeida, L.; Stüve, P.; Velasquez, L.; Dhillon-LaBrooy, A.; Roy, U.; Lindenberg, M.; Strowig, T.; Plaza-Sirvent, C.; Schmitz, I.; et al. Regulating T-cell differentiation through the polyamine spermidine. J. Allergy Clin. Immunol. 2021, 147, 335–348. [Google Scholar] [CrossRef]
- Guo, F.-F.; Meng, F.-G.; Zhang, X.-N.; Zeng, T. Spermidine inhibits LPS-induced pro-inflammatory activation of macrophages by acting on Nrf2 signaling but not autophagy. J. Funct. Foods 2022, 94, 105115. [Google Scholar] [CrossRef]
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.; de Sousa, D.P. An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxid. Med. Cell. Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef]
- Aburel, O.M.; Pavel, I.Z.; Dănilă, M.D.; Lelcu, T.; Roi, A.; Lighezan, R.; Muntean, D.M.; Rusu, L.C. Pleiotropic effects of eugenol: The good, the bad, and the unknown. Oxid. Med. Cell. Longev. 2021, 2021, 3165159. [Google Scholar] [CrossRef]
- Sun, X.; Wang, D.; Zhang, T.; Lu, X.; Duan, F.; Ju, L.; Zhuang, X.; Jiang, X. Eugenol attenuates cerebral ischemia-reperfusion injury by enhancing autophagy via AMPK-mTOR-P70S6K pathway. Front. Pharmacol. 2020, 11, 84. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.M.; Mohamad Hanif, E.A.; Chin, S.F. Is targeting autophagy mechanism in cancer a good approach? The possible double-edge sword effect. Cell Biosci. 2021, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.N.; Xiao, L.; Wang, J.Y. Polyamines in gut epithelial renewal and barrier function. Physiology 2017, 35, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Vucicevic, L.; Misirkic, M.; Janjetovic, K.; Vilimanovich, U.; Sudar, E.; Isenovic, E.; Prica, M.; Harhaji-Trajkovic, L.; Kravic-Stevovic, T.; Bumbasirevic, V.; et al. Compound C induces protective autophagy in cancer cells through AMPK inhibition-independent blockade of Akt/mTOR pathway. Autophagy 2011, 7, 40–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.T.; Qin, Y.; Yao, G.D.; Tian, Z.N.; Fu, S.B.; Geng, J.S. Astrocyte elevated gene-1 mediates glycolysis and tumorigenesis in colorectal carcinoma cells via AMPK signaling. Mediat. Inflamm. 2014, 2014, 287381. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, V.; Ferlazzo, N.; Currò, M.; Isola, G.; Matarese, M.; Bertuccio, M.P.; Caccamo, D.; Matarese, G.; Ientile, R. Baicalin-induced autophagy preserved LPS-Stimulated intestinal cells from inflammation and alterations of paracellular permeability. Int. J. Mol. Sci. 2021, 22, 2315. [Google Scholar] [CrossRef]
- Bao, M.; Liang, M.; Sun, X.; Mohyuddin, S.G.; Chen, S.; Wen, J.; Yong, Y.; Ma, X.; Yu, Z.; Ju, X.; et al. Baicalin Alleviates LPS-Induced Oxidative Stress via NF-κB and Nrf2-HO1 Signaling Pathways in IPEC-J2 Cells. Front. Vet. Sci. 2022, 8, 808233. [Google Scholar] [CrossRef]
- Guo, S.; Nighot, M.; Al-Sadi, R.; Alhmoud, T.; Nighot, P.; Ma, T.Y. Lipopolysaccharide Regulation of Intestinal Tight Junction Permeability Is Mediated by TLR4 Signal Transduction Pathway Activation of FAK and MyD88. J. Immunol. 2015, 195, 4999–5010. [Google Scholar] [CrossRef] [Green Version]
- Nighot, M.; Al-Sadi, R.; Guo, S.; Rawat, M.; Nighot, P.; Watterson, M.D.; Ma, T.D. Lipopolysaccharide-induced increase in intestinal epithelial tight permeability is mediated by toll-like receptor 4/myeloid differentiation primary response 88 (MyD88) Activation of myosin light chain kinase expression. Am. J. Pathol. 2017, 187, 2698–2710. [Google Scholar] [CrossRef] [Green Version]
- Orhon, I.; Reggiori, F. Assays to monitor autophagy progression in cell cultures. Cells 2017, 6, 20. [Google Scholar] [CrossRef] [Green Version]
- Ney, P.A. Mitochondrial autophagy: Origins, significance, and role of BNIP3 and NIX. Biochim. Biophys. Acta 2015, 1853 Pt B, 2775–2783. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhang, J.; Liu, C.; Wang, Q.; Yan, J.; Hui, L.; Jia, Q.; Shan, H.; Tao, L.; Zhang, M. The role of mitophagy in regulating cell death. Oxid. Med. Cell. Longev. 2021, 2021, 6617256. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, L.P.; Zapata-Muñoz, J.; Villarejo-Zori, B.; Pellegrin, S.; Freire, C.M.; Toye, A.M.; Boya, P.; Ganley, I.G. BNIP3L/NIX regulates both mitophagy and pexophagy. EMBO J. 2022, 41, e111115. [Google Scholar] [CrossRef] [PubMed]
- Kekilli, M.; Tanoğlu, A.; Karaahmet, F.; Doğan, Z.; Can, M.; Sayilir, A.; Çakal, B.; Düzenli, T.; Beyazit, Y. Midkine level may be used as a noninvasive biomarker in Crohn’s disease. Turk. J. Med. Sci. 2020, 50, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Lian, P.; Braber, S.; Varasteh, S.; Wichers, H.J.; Folkerts, G. Hypoxia and heat stress affect epithelial integrity in a Caco-2/HT-29 co-culture. Sci. Rep. 2021, 11, 13186. [Google Scholar] [CrossRef]
- Federal Register. Eugenol: Exemption from the Requirement of a Tolerance. 2022. Available online: https://www.federalregister.gov/documents/2022/09/16/2022-20041/eugenol-exemption-from-the-requirement-of-a-tolerance (accessed on 18 December 2022).
- Khalil, A.A.; Rahman, U.; Khan, M.R.; Sahar, A.; Mehmood, T.; Khan, M. Essential oil eugenol: Sources, extraction techniques and nutraceutical perspectives. RSC Adv. 2017, 7, 32669–32681. [Google Scholar] [CrossRef] [Green Version]
- Nisar, M.F.; Khadim, M.; Rafiq, M.; Chen, J.; Yang, Y.; Wan, C.C. Pharmacological properties and health benefits of eugenol: A Comprehensive Review. Oxid. Med. Cell. Longev. 2021, 2021, 2497354. [Google Scholar] [CrossRef]
- Bein, A.; Zilbershtein, A.; Golosovsky, M.; Davidov, D.; Schwartz, B. LPS induces hyper-permeability of intestinal epithelial cells. J. Cell. Physiol. 2017, 232, 381–390. [Google Scholar] [CrossRef]
- Wu, J.; He, C.; Bu, J.; Luo, Y.; Yang, S.; Ye, C.; Yu, S.; He, B.; Yin, Y.; Yang, X. Betaine attenuates LPS-induced downregulation of Occludin and Claudin-1 and restores intestinal barrier function. BMC Vet. Res. 2020, 16, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornick, S.; Tawiah, A.; Chadee, K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers 2015, 3, e982426. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Rao, J.N.; Liu, L.; Zou, T.; Keledjian, K.M.; Boneva, D.; Marasa, B.S.; Wang, J.Y. Polyamines are necessary for synthesis and stability of occludin protein in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G1159–G1169. [Google Scholar] [CrossRef] [Green Version]
- Hui, Q.; Ammeter, E.; Liu, S.; Yang, R.; Lu, P.; Lahaye, L.; Yang, C. Eugenol attenuates inflammatory response and enhances barrier function during lipopolysaccharide-induced inflammation in the porcine intestinal epithelial cells. J. Anim. Sci. 2020, 98, skaa245. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zheng, S.; Wei, S.; Tian, Q.; Tao, Y.; Bo, R.; Liu, M.; Li, J. The protective effect and potential mechanisms of eugenol against Salmonella in vivo and in vitro. Poult. Sci. 2022, 101, 101801. [Google Scholar] [CrossRef] [PubMed]
- van Klinken, B.J.; Oussoren, E.; Weenink, J.J.; Strous, G.J.; Büller, H.A.; Dekker, J.; Einerhand, A.W. The human intestinal cell lines Caco-2 and LS174T as models to study cell-type specific mucin expression. Glycoconj. J. 1996, 13, 757–768. [Google Scholar] [CrossRef]
- Ma, L.; Liu, J.; Lin, Q.; Gu, Y.; Yu, W. Eugenol protects cells against oxidative stress via Nrf2. Exp. Ther. Med. 2021, 21, 107. [Google Scholar] [CrossRef]
- Popelka, H.; Klionsky, D.J. The molecular mechanism of Atg13 function in autophagy induction: What is hidden behind the data? Autophagy 2017, 13, 449–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Yao, X.; Zhang, Q.J.; Zhu, M.; Liu, Z.P.; Ci, B.; Xie, Y.; Carlson, D.; Rothermel, B.A.; Sun, Y.; et al. Beclin-1-Dependent Autophagy Protects the Heart During Sepsis. Circulation 2018, 138, 2247–2262. [Google Scholar] [CrossRef]
- Huynh, K.K.; Eskelinen, E.L.; Scott, C.C.; Malevanets, A.; Saftig, P.; Grinstein, S. LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J. 2007, 26, 313–324. [Google Scholar] [CrossRef]
- Vincent, G.; Novak, E.A.; Siow, V.S.; Cunningham, K.E.; Griffith, B.D.; Comerford, T.E.; Mentrup, H.L.; Stolz, D.B.; Loughran, P.; Ranganathan, S.; et al. Nix-mediated mitophagy modulates mitochondrial damage during intestinal inflammation. Antioxid. Redox Signal. 2020, 33, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Choubey, V.; Zeb, A.; Kaasik, A. Molecular mechanisms and regulation of mammalian mitophagy. Cells 2022, 11, 38. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truzzi, F.; Whittaker, A.; D’Amen, E.; Valerii, M.C.; Abduazizova, V.; Spisni, E.; Dinelli, G. Spermidine-Eugenol Supplement Preserved Inflammation-Challenged Intestinal Cells by Stimulating Autophagy. Int. J. Mol. Sci. 2023, 24, 4131. https://doi.org/10.3390/ijms24044131
Truzzi F, Whittaker A, D’Amen E, Valerii MC, Abduazizova V, Spisni E, Dinelli G. Spermidine-Eugenol Supplement Preserved Inflammation-Challenged Intestinal Cells by Stimulating Autophagy. International Journal of Molecular Sciences. 2023; 24(4):4131. https://doi.org/10.3390/ijms24044131
Chicago/Turabian StyleTruzzi, Francesca, Anne Whittaker, Eros D’Amen, Maria Chiara Valerii, Veronika Abduazizova, Enzo Spisni, and Giovanni Dinelli. 2023. "Spermidine-Eugenol Supplement Preserved Inflammation-Challenged Intestinal Cells by Stimulating Autophagy" International Journal of Molecular Sciences 24, no. 4: 4131. https://doi.org/10.3390/ijms24044131
APA StyleTruzzi, F., Whittaker, A., D’Amen, E., Valerii, M. C., Abduazizova, V., Spisni, E., & Dinelli, G. (2023). Spermidine-Eugenol Supplement Preserved Inflammation-Challenged Intestinal Cells by Stimulating Autophagy. International Journal of Molecular Sciences, 24(4), 4131. https://doi.org/10.3390/ijms24044131