New Thienobenzo/Naphtho-Triazoles as Butyrylcholinesterase Inhibitors: Design, Synthesis and Computational Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of New Thienobenzo/Naphtho-Triazoles 1–19
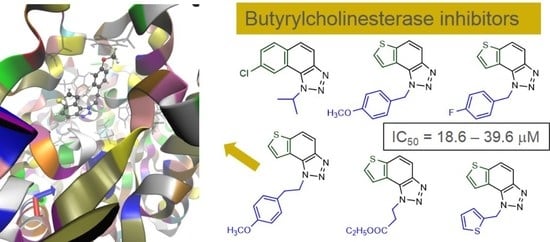
2.2. Inhibitory Activity of Thienobenzo/Naphtho-Triazoles 1–19 toward Enzymes Cholinesterases
2.3. Computational Study of Thienobenzo/Naphtho-Triazoles 1–19 as Cholinesterase Inhibitors
2.4. Crystal Structure of Compound 5
3. Materials and Methods
3.1. General Remarks
3.2. General Procedure for the Synthesis of Starting Compounds 1a–6a
3.3. General Procedure for the Synthesis of the Electrocyclization Photoproducts 1–6

3.4. General Procedure for the Synthesis of the Thienobenzo-Triazoles 7–12




3.5. Cholinesterase Inhibition Activity Measurements
3.6. Computational Details
3.7. X-ray Diffraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, P.; Radić, Z. The cholinesterases: From genes to proteins. Annu. Rev. Pharmacol. Toxicol. 1994, 33, 281–320. [Google Scholar] [CrossRef] [PubMed]
- Sussman, J.L.; Harel, M.; Frolow, F.; Oefner, C.; Goldman, A.; Toker, L.; Silman, I. Atomic structure of acetylcholinesterase from Torpedo Californica: A prototypic acetylcholine-binding protein. Science 1991, 253, 872–897. [Google Scholar] [CrossRef]
- Kovarik, Z. Amino acid residues conferring specificity of cholinesterases. Period. Biol. 1999, 101, 7–15. [Google Scholar]
- Radić, Z.; Pickering, N.A.; Vellom, D.C.; Camp, S.; Taylor, P. Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase inhibitors. Biochemistry 1993, 32, 12074–12084. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Redman, A.M.G.; Jiang, X.; Lockridge, O.; Doctor, B.P. Differences in active-site gorge dimensions of cholinesterase revealed by binding of inhibitors to human butyrylcholinesterase. Chem. Biol. Interact. 1999, 119–120, 61–69. [Google Scholar] [CrossRef]
- Taylor, P.; Radić, Z.; Hosea, N.A.; Camp, S.; Marchot, P.; Berman, H.A. Structural bases for the specificity of cholinesterase catalysis and inhibition. Toxicol. Lett. 1995, 82−83, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Soreq, H.; Seidman, S. Acetylcholinesterase—New roles for an old actor. Nat. Rev. Neurosci. 2001, 2, 294–302. [Google Scholar] [CrossRef]
- Silman, I.; Sussman, J.L. Acetylcholinesterase: ‘Classical’ and ‘non-classical’ functions and pharmacology. Curr. Opin. Pharmacol. 2005, 5, 293–302. [Google Scholar] [CrossRef]
- Pienica, C.; Soreq, H. MicroRNA regulators of cholinergic signaling link neuromuscular, cardiac and metabolic systems. Period. Biol. 2016, 118, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Chatonnet, A.; Lockridge, O. Comparison of butyrylcholinesterase and acetylcholinesterase. Biochem. J. 1989, 260, 625–634. [Google Scholar] [CrossRef]
- Çokugras, A.N. Butyrylcholinesterase: Structure and physiological importance. Turk. J. Biochem. 2003, 28, 54–61. [Google Scholar]
- Mesulam, M.M.; Guillozet, A.; Shaw, P.; Levey, A.; Duysen, E.G.; Lockridge, O. Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience 2002, 110, 627–639. [Google Scholar] [CrossRef]
- Guillozet, A.L.; Smiley, J.F.; Mash, D.C. Butyrylcholinesterase in the life cycle of amyloid plaques. Ann. Neurol. 1997, 42, 909–918. [Google Scholar] [CrossRef]
- Mesulam, M.M.; Geula, C. Butyrylcholinesterase reactivity differentiates the amyloid plaques of aging from those of dementia. Ann. Neurol. 1994, 36, 722–727. [Google Scholar] [CrossRef]
- Darvesh, S.; Walsh, R.; Kumar, R.; Caines, A.; Roberts, S.; Magee, D.; Rockwood, K.; Martin, E. Inhibition of human cholinesterases by drugs used to treat Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2003, 17, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Šagud, I.; Maček Hrvat, N.; Grgičević, A.; Čadež, T.; Hodak, J.; Dragojević, M.; Lasić, K.; Kovarik, Z.; Škorić, I. Design, synthesis and cholinesterase inhibitory properties of new oxazole benzylamine derivatives. J. Enzyme Inhib. Med. Chem. 2020, 1, 460–467. [Google Scholar] [CrossRef]
- Bosak, A.; Opsenica, D.M.; Šinko, G.; Zlatar, M.; Kovarik, Z. Structural aspects of 4-aminoquinolines as reversible inhibitors of human acetylcholinesterase and butyrylcholinesterase. Chem. Biol. Interact. 2019, 308, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Mamedova, G.; Mahmudova, A.; Mamedov, S.; Erden, Y.; Taslimi, P.; Tüzün, B.; Tas, R.; Farzaliyev, V.; Sujayev, A.; Alwasel, S.H.; et al. Novel tribenzylaminobenzolsulphonylimine based on their pyrazine and pyridazines: Synthesis, characterization, antidiabetic, anticancer, anticholinergic, and molecular docking studies. Bioorg. Chem. 2019, 93, 103313. [Google Scholar] [CrossRef]
- Katalinić, M.; Bosak, A.; Kovarik, Z. Flavonoids as inhibitors of human butyrylcholinesterase variants. Food Technol. Biotechnol. 2014, 52, 64–67. [Google Scholar]
- El-Sayed, N.A.E.; El-Said Farag, A.; Ezzat, M.A.F.; Akincioglu, H.; Gülçin, I.; Abou-Seri, S.M. Design, synthesis, in vitro and in vivo evaluation of novel pyrrolizine-based compounds with potential activity as cholinesterase inhibitors and anti-Alzheimer’s agents. Bioorg. Chem. 2019, 93, 103312. [Google Scholar] [CrossRef]
- Mlakić, M.; Odak, I.; Faraho, I.; Talić, S.; Bosnar, M.; Lasić, K.; Barić, D.; Škorić, I. New naphtho/thienobenzo-triazoles with interconnected anti-inflammatory and cholinesterase inhibitory activity. Eur. J. Med. Chem. 2022, 241, 114616. [Google Scholar] [CrossRef]
- Mlakić, M.; Faraho, I.; Odak, I.; Talić, S.; Vukovinski, A.; Raspudić, A.; Bosnar, M.; Zadravec, R.; Ratković, A.; Lasić, K.; et al. Synthesis, photochemistry and computational study of novel 1,2,3-triazole heterostilbenes: Expressed biological activity of their electrocyclization photoproducts. Bioorg. Chem. 2022, 121, 105701. [Google Scholar] [CrossRef] [PubMed]
- Atatreh, N.; Al Rawashdah, S.; Al Neyadi, S.S.; Abuhamdah, S.; Ghattas, M.A. Discovery of new butyrylcholinesterase inhibitors via structure-based virtual screening. J. Enz. Inh. Med. Chem. 2019, 34, 1373–1379. [Google Scholar] [CrossRef] [Green Version]
- Opsomer, T.; Valkeneers, K.; Ratković, A.; Dehaen, W. 1-(4-Nitrophenyl)-1H-1,2,3-triazole-4-carbaldehyde: Scalable synthesis and its use in the preparation of 1-alkyl-4-formyl-1,2,3-triazoles. Organics 2021, 2, 404–414. [Google Scholar] [CrossRef]
- Singh, H.; Khanna, G.; Khurana, J.M. DBU catalyzed metal-free synthesis of fused 1,2,3-triazoles through [3+2] cycloaddition of aryl azides with activated cyclic C–H acids. Tetrahedron Lett. 2016, 57, 3075–3080. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, H.; Chen, H.; Deng, G.-J. Catalyst- and additive-free annulation/aromatization leading to benzothiazoles and naphthothiazoles. Org. Chem. Front. 2019, 6, 3060. [Google Scholar] [CrossRef]
- Horsten, T.; De Jong, F.; Theunissen, D.; Van der Auweraer, M.; Dehaen, W. Synthesis and spectroscopic properties of 1,2,3-triazole BOPAHY dyes and their water-soluble triazolium salts. J. Org. Chem. 2021, 86, 13774–13782. [Google Scholar] [CrossRef]
- Giacobini, E. Cholinesterase inhibitors: New roles and therapeutic alternatives. Pharmacol. Res. 2004, 50, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Bourne, Y.; Kolb, H.C.; Radić, Z.; Sharpless, K.B.; Taylor, P.; Marchot, P. Freeze-frame inhibitor captures acetylcholinesterase in a unique conformation. Proc. Natl. Acad. Sci. USA 2004, 101, 1449–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galdeano, C.; Coquelle, N.; Cieslikiewicz-Bouet, M.; Bartolini, M.; Pérez, B.; Clos, M.V.; Silman, I.; Jean, L.; Colletier, J.-P.; Renard, P.-Y.; et al. Increasing polarity in tacrine and huprine derivatives: Potent anticholinesterase agents for the treatment of myasthenia gravis. Molecules 2018, 23, 634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourne, Y.; Radić, Z.; Taylor, P.; Marchot, P. Conformational remodeling of femtomolar inhibitor−acetylcholinesterase complexes in the crystalline ctate. J. Am. Chem. Soc. 2010, 132, 18292–18300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourne, Y.; Sharpless, K.B.; Taylor, P.; Marchot, P. Steric and dynamic parameters influencing in situ cycloadditions to form triazole inhibitors with crystalline acetylcholinesterase. J. Am. Chem. Soc. 2016, 138, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.N.; Inoue, Y.; Nakanishi, I.; Kitaura, K. Cl–pi interactions in protein–ligand complexes. Protein Sci. 2008, 17, 1129–1137. [Google Scholar] [CrossRef] [Green Version]
- Ellman, G.L.; Courtnex, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDock-823 Tools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Raves, M.L.; Giles, K.; Schrag, J.D.; Schmid, M.F.; Phillips, G.N., Jr.; Wah, C.; Howard, A.J.; Silman, I.; Sussman, J.L. Quaternary structure of tetrameric acetylcholinesterase. In Structure and Function of Cholinesterases and Related Proteins; Doctor, B.P., Quinn, D.M., Rotundo, R.L., Taylor, P., Eds.; Springer: Boston, MA, USA, 1998; pp. 351–356. [Google Scholar]
- Nicolet, Y.; Lockridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J. Biol. Chem. 2003, 278, 41141–41147. [Google Scholar] [CrossRef] [Green Version]
- Rigaku, O.D. P.R.O. CrysAlis, version: 1.171.39.46; Rigaku Oxford Diffraction Ltd.: Yarnton, UK, 2018. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spek, A.L. checkCIF validation ALERTS: What they mean and how to respond. Acta Grystallogr. E 2020, 76, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrugia, L.J. ORTEP-3 for Windows—A version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Compound 1 | Structure | IC50 (μM) AChE | IC50 (μM) BChE | Compound | Structure | IC50 (μM) AChE | IC50 (μM) BChE |
|---|---|---|---|---|---|---|---|
| 1 |  | 59.9 | 39.6 | 12 |  | - | 106.7 |
| 3 |  | 116.0 | 25.5 | 13 |  | - | 58.0 |
| 4 |  | 97.0 | 37.5 | 14 |  | 117.6 | >150 |
| 5 |  | 118.6 | 23.4 | 15 |  | 88.7 | - |
| 6 |  | - | 39.6 | 16 |  | 145.3 | 119.4 |
| 7 |  | 139.6 | >150 | 17 |  | 45.6 | - |
| 8 |  | 68.0 | 213.5 | 19 |  | - | 74.1 |
| 9 |  | - | 18.6 | Galantamine | 0.15 | 7.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlakić, M.; Selec, I.; Ćaleta, I.; Odak, I.; Barić, D.; Ratković, A.; Molčanov, K.; Škorić, I. New Thienobenzo/Naphtho-Triazoles as Butyrylcholinesterase Inhibitors: Design, Synthesis and Computational Study. Int. J. Mol. Sci. 2023, 24, 5879. https://doi.org/10.3390/ijms24065879
Mlakić M, Selec I, Ćaleta I, Odak I, Barić D, Ratković A, Molčanov K, Škorić I. New Thienobenzo/Naphtho-Triazoles as Butyrylcholinesterase Inhibitors: Design, Synthesis and Computational Study. International Journal of Molecular Sciences. 2023; 24(6):5879. https://doi.org/10.3390/ijms24065879
Chicago/Turabian StyleMlakić, Milena, Ida Selec, Irena Ćaleta, Ilijana Odak, Danijela Barić, Ana Ratković, Krešimir Molčanov, and Irena Škorić. 2023. "New Thienobenzo/Naphtho-Triazoles as Butyrylcholinesterase Inhibitors: Design, Synthesis and Computational Study" International Journal of Molecular Sciences 24, no. 6: 5879. https://doi.org/10.3390/ijms24065879
APA StyleMlakić, M., Selec, I., Ćaleta, I., Odak, I., Barić, D., Ratković, A., Molčanov, K., & Škorić, I. (2023). New Thienobenzo/Naphtho-Triazoles as Butyrylcholinesterase Inhibitors: Design, Synthesis and Computational Study. International Journal of Molecular Sciences, 24(6), 5879. https://doi.org/10.3390/ijms24065879


_Kim.png)