Metabolic Composition and Quality Traits of Polygonatum cyrtonema Hua from Different Germplasms and Age Sections Based on Widely Targeted Metabolomics Analysis
Abstract
:1. Introduction
2. Results
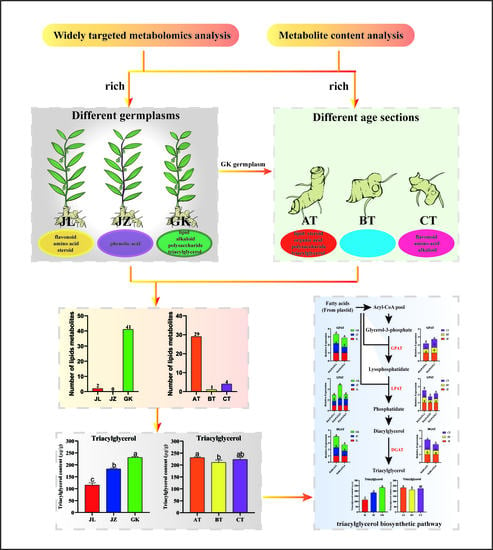
2.1. Phenotypic Variations among Different P. cyrtonema Germplasms
2.2. Widely Targeted Metabolomic Analysis of Rhizomes from Different Germplasms of P. cyrtonema
2.3. KEGG Enrichment Analysis to Determine the Differences in Lipid Metabolites among Rhizomes from Different Germplasms of P. cyrtonema
2.4. Widely Targeted Metabolomics Analysis of Rhizome Sections of Different Ages Sections of P. cyrtonema
2.5. KEGG Enrichment Analysis of Differential Metabolites of P. cyrtonema Rhizome Sections of Different Ages
2.6. Determination of the Triacylglycerol and Polysaccharide Contents of Rhizomes of Different Germplasms and Age Sections and Analysis of Triacylglycerol Synthesis-Related Genes in P. cyrtonema
3. Discussion
3.1. Three P. cyrtonema Germplasms with Different Nutritional and Medicinal Components
3.2. One-Year and Three-Year P. cyrtonema Rhizomes Have Higher Functional Value
3.3. Broad-Leaved Green Stem Germplasm and One-Year-Old P. cyrtonema Sections May Exhibit Higher Resistance
4. Materials and Methods
4.1. Plant Materials
4.2. Sample Pretreatment
4.3. UPLC-MS/MS Analysis
4.4. Triacylglycerol and Polysaccharide Content Analysis
4.5. Triacylglycerol Synthesis-Related Gene Screening Based on Transcriptome Data
4.6. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Z.; Du, X.; Ma, C.; Zhang, R.; Gong, W.; Liu, F. Identification of antitumor active constituents in Polygonatum sibiricum flower by UPLC-Q-TOF-MSE and network pharmacology. ACS Omega 2020, 5, 29755–29764. [Google Scholar] [CrossRef]
- Shen, F.; Song, Z.; Xie, P.; Li, L.; Wang, B.; Peng, D.; Zhu, G. Polygonatum sibiricum polysaccharide prevents depression-like behaviors by reducing oxidative stress, inflammation, and cellular and synaptic damage. J. Ethnopharmacol. 2021, 275, 114164. [Google Scholar] [CrossRef]
- Long, T.; Liu, Z.; Shang, J.; Zhou, X.; Yu, S.; Tian, H.; Bao, Y. Polygonatum sibiricum polysaccharides play anti-cancer effect through TLR4-MAPK/NF-Kappa B signaling pathways. Int. J. Biol. Macromol. 2018, 111, 813–821. [Google Scholar] [CrossRef]
- Wang, S.; Li, G.; Zhang, X.; Wang, Y.; Qiang, Y.; Wang, B.; Zou, J.; Niu, J.; Wang, Z. Structural characterization and antioxidant activity of Polygonatum sibiricum polysaccharides. Carbohyd. Polym. 2022, 291, 119524. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, W.; Liu, W.; Bai, J.; Xie, S.; Wang, T.; Xu, G.; Wu, D. Physicochemical characterization and hypoglycemic potential of a novel polysaccharide from Polygonatum sibiricum Red through PI3K/Akt mediated signaling pathway. J. Funct. Foods 2022, 93, 105080. [Google Scholar] [CrossRef]
- Wang, S. Glycomics, Metabolomics and Transcriptomics-Based Research on Polygonatum sibiricum Red Germplasm Resources. Ph.D. Thesis, Shaanxi Normal University , Xi’an, China, 2017; p. 200. [Google Scholar]
- Zhang, J.; Qiu, X.; Tan, Q.; Xiao, Q.; Mei, S. A comparative metabolomics study of flavonoids in radish with different skin and flesh colors (Raphanus sativus L.). J. Agric. Food Chem. 2020, 68, 14463–14470. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, X.; Chen, X.; Xu, Y.; Liu, J.; Tan, J.; Li, W.; Tembrock, L.R.; Wu, Z.; Zhu, G. The use of widely targeted metabolomics profiling to quantify differences in medicinally important compounds from five Curcuma (Zingiberaceae) species. Ind. Crop. Prod. 2022, 175, 114289. [Google Scholar] [CrossRef]
- Wang, S.; Li, W.; Zhang, X.; Li, G.; Li, X.D.; Chang, H.; Niu, J.; Wang, Z. Metabolomics Study of Different Germplasm Resources for Three Polygonatum Species Using UPLC-Q-TOF-MS/MS. Front. Plant Sci. 2022, 13, 826902. [Google Scholar] [CrossRef]
- Hu, Y.; Yin, M.; Bai, Y.; Chu, S.; Zhang, L.; Yang, M.; Zheng, X.; Yang, Z.; Liu, J.; Li, L.; et al. An evaluation of traits, nutritional, and medicinal component quality of Polygonatum cyrtonema Hua and P. sibiricum Red. Front. Plant Sci. 2022, 13, 891775. [Google Scholar] [CrossRef]
- Zhao, Q. Study on Quality Evaluation of Wild Polygonatum cyrtonema Hua in Different Populations and Ages in Anhui Province. MSc Thesis, Wenzhou University, Wenzhou, China, 2019; p. 68. [Google Scholar] [CrossRef]
- Song, F.; Huang, Z.; Luo, Z.; Zhang, P.; Lin, A.; Sun, D.; Meng, Y. Metabolomics and its application in medicinal plants. J. S.-Cent. Univ. Natl. (Nat. Sci. Ed.). 2016, 35, 36–41. [Google Scholar] [CrossRef]
- Yuji, S.; Kenji, A.; Akane, S.; Ayuko, K.; Hitomi, O.; Tetsuya, S.; Kazuki, S.; Yokota, H.M. Widely targeted metabolomics based on large-scale MS/MS data for elucidating metabolite accumulation patterns in plants. Plant Cell Physiol. 2009, 50, 37–47. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A Novel Integrated Method for Large-Scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Zhang, L.; Huang, X.; Wang, X.; Yang, R.; Mao, J.; Wang, X.; Wang, X.; Zhang, Q.; Li, P. Identification of Nutritional Components in Black Sesame Determined by Widely Targeted Metabolomics and Traditional Chinese Medicines. Molecules 2018, 23, 1180. [Google Scholar] [CrossRef] [Green Version]
- Nam, J.; Lee, H.G.; Do, H.; Kim, H.U.; Seo, P.J. Transcriptional regulation of triacylglycerol accumulation in plants under environmental stress conditions. J. Exp. Bot. 2022, 73, 2905–2917. [Google Scholar] [CrossRef]
- Chapman, K.D.; Ohlrogge, J.B. Compartmentation of triacylglycerol accmulation in plants. J. Biol. Chem. 2012, 287, 2288–2294. [Google Scholar] [CrossRef] [Green Version]
- Snaebjornsson, M.T.; Janaki-Raman, S.; Schulze, A. Greasing the Wheels of the Cancer Machine: The Role of Lipid Metabolism in Cancer. Cell Metab. 2020, 31, 62–76. [Google Scholar] [CrossRef]
- Zou, S.; Wu, J.; Shahid, M.Q.; He, Y.; Lin, S.; Liu, Z.; Yang, X. Identification of key taste components in loquat using widely targeted metabolomics. Food Chem. 2020, 323, 126822. [Google Scholar] [CrossRef]
- Xiao, J.; Gu, C.; He, S.; Zhu, D.; Huang, Y.; Zhou, Q. Widely targeted metabolomics analysis reveals new biomarkers and mechanistic insights on chestnut (Castanea mollissima Bl.) calcification process. Food Res. Int. 2021, 141, 110128. [Google Scholar] [CrossRef]
- Feng, Z.; Gao, Z.; Jiao, X.; Shi, J.; Wang, R. Widely targeted metabolomic analysis of active compounds at different maturity stages of ‘Hupingzao’ jujube. J. Food Compos. Anal. 2020, 88, 103417. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef] [Green Version]
- Watt, M.J.; Cheng, Y. Triglyceride metabolism in exercising muscle. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2017, 1862, 1250–1259. [Google Scholar] [CrossRef]
- Fan, Z.; Ke, X.; Chen, L.; Meng, Y. Advances in chemical constituents and pharmacological acitivies of Polygonatum cyrtonema Hua. Inf. Tradit. Chin. Med. 2020, 37, 119–126. [Google Scholar] [CrossRef]
- Piletz, J.E.; Aricioglu, F.; Cheng, J.; Fairbanks, C.A.; Gilad, V.H.; Haenisch, B.; Halaris, A.; Hong, S.; Lee, J.E.; Li, J.; et al. Agmatine: Clinical applications after 100 years in translation. Drug Discov. Today 2013, 18, 880–893. [Google Scholar] [CrossRef]
- Ashihara, H.; Ludwig, I.A.; Katahira, R.; Yokota, T.; Fujimura, T.; Crozier, A. Trigonelline and related nicotinic acid metabolites: Occurrence, biosynthesis, taxonomic considerations, and their roles in planta and in human health. Phytochem. Rev. 2015, 14, 765–798. [Google Scholar] [CrossRef]
- Tao, A.; Zhang, X.; Du, Z.; Zhao, F.; Duan, B. Research progress on flavonoids in plants of Polygonatum Mill. and their pharmacological activities. Chin. Tradit. Herbal Drugs 2018, 49, 2163–2171. [Google Scholar]
- Jiang, T.; Wu, T.; Gao, P.; Wang, L.; Yang, X.; Chen, X.; Chen, Y.; Yue, C.; Liang, K.; Tang, L.; et al. Research on processing-induced chemical variations in Polygonatum Cyrtonema rhizome by integrating metabolomics and glycomics. Molecules 2022, 27, 5869. [Google Scholar] [CrossRef]
- Choi, S.; Ahn, J.; Kim, H.; Im, N.; Kozukue, N.; Levin, C.E.; Friedman, M. Changes in free amino acid, Protein, and flavonoid content in Jujube (Ziziphus jujube) fruit during eight stages of growth and antioxidative and cancer cell inhibitory effects by extracts. J. Agric. Food chem. 2012, 60, 10245–10255. [Google Scholar] [CrossRef]
- Wang, J.; Ding, H. Advances in the study of bacterial inhibition by phenolic acid compounds. Chin. Tradit. Pat. Med. 2022, 44, 1906–1911. [Google Scholar] [CrossRef]
- Li, D.; Wang, Q.; Chen, S.; Liu, H.; Pan, K.; Li, J.; Luo, C.; Wang, H. De novo assembly and analysis of Polygonatum cyrtonema Hua and identification of genes involved in polysaccharide and saponin biosynthesis. BMC Genom. 2022, 23, 195. [Google Scholar] [CrossRef]
- Jiang, W.; Weng, G.; Chen, J.; Ye, C.; Jiang, X.; Tao, Z. Comparative analysis of chemical constituents of Polygonatum cyrtonema Hua of red and green stem type based on LC-MS metabolomics. Chin. Agric. Sci. Bull. 2021, 37, 32–38. [Google Scholar] [CrossRef]
- Miao, X.; Song, G.; Liu, R.; Li, X.; Xu, L.; Wang, W.; Zhang, X.; Wei, S. Effect of different cultivation years and harvesting period of Scutellaria baicalensis on active ingredients. Mod. Chin. Med. 2015, 17, 836–839. [Google Scholar] [CrossRef]
- Tang, X.; Liu, J.; Li, L. Pharmacological effects of organic acids in chinese herbs and its application in cardiovascular diseases. Chin. J. Exp. Tradit. Med. Form. 2012, 18, 243–246. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, Y.; Zhao, Y. Comparison study of polysaccharide content and material accumulation in wild Polygonatum cyrtonema Hua and Polygonatum filipes Merr. in Fujian. In Proceedings of the Chinese Medicine and Natural Medicines Summit and the 12th National Symposium on Chinese Medicine and Natural Medicines, Haikou, China, 2 November 2012; pp. 109–112. [Google Scholar]
- Chen, Y.; Yao, Y.; Chen, S.; Liu, H.; Zhao, Z. Medicinal quality relating to age of Polygonatum cyrtonema Hua plant sections. Fujian J. Agric. Sci. 2020, 35, 38–43. [Google Scholar] [CrossRef]
- Gidda, S.K.; Jay, M.S.; Steven, J.R.; John, M.D.; Mullen, R.T. Arabidopsis thaliana GPAT8 and GPAT9 are localized to the ER and possess distinct ER retrieval signals: Functional divergence of the dilysine ER retrieval motif in plant cells. Plant Physiol. Bioch. 2009, 47, 867–879. [Google Scholar] [CrossRef]
- Moellering, E.R.; Muthan, B.; Benning, C. Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science 2010, 330, 226–228. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Xu, Y.; Wang, J.; Singer, S.D.; Chen, G. The Role of Triacylglycerol in Plant Stress Response. Plants 2020, 9, 472. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Francis, T.; Mietkiewska, E.; Giblin, E.M.; Barton, D.L.; Zhang, Y.; Zhang, M.; Taylor, D.C. Cloning and characterization of an acyl-CoA-dependentdiacylglycerol acyltransferase 1 (DGAT1) gene from Tropaeolum majus, and a study of the functional motifs of the DGAT protein using site-directed mutagenesis to modify enzyme activity and oil content. Plant Biotechnol. J. 2008, 6, 799–818. [Google Scholar] [CrossRef]
- Chen, S.; Lei, Y.; Xu, X.; Huang, J.; Jiang, H.; Wang, J.; Cheng, Z.; Zhang, J.; Song, Y.; Liao, B.; et al. The peanut (Arachis hypogaea L.) gene AhLPAT2 increases the lipid content of transgenic Arabidopsis seeds. PLoS ONE 2015, 10, e136170. [Google Scholar] [CrossRef] [Green Version]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China: Part 1; China Medical Science Press: Beijing, China, 2020; pp. 319–320.
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcrip tome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Koressaar, T.; Lepamets, M.; Kaplinski, L.; Raime, K.; Andreson, R.; Remm, M. Primer3_masker: Integrating masking of template sequence with primer design software. Bioinformatics 2018, 34, 1937–1938. [Google Scholar] [CrossRef] [Green Version]
- Wang, W. The Molecular Detection of Corynespora Cassiicola on Cucumber by PCR Assay Using DNAman Software and NCBI. In Computer and Computing Technologies in Agriculture IX: 9th IFIP WG 5.14 International Conference, CCTA 2015, Beijing, China, September 27-30, 2015, Revised Selected Papers, Part II 9; Li, D., Li, Z., Eds.; Springer International Publishing AG: Cham, Switzerland, 2016; Volume 479, pp. 248–258. [Google Scholar]
- Yang, Y.; Ye, B.; Song, Q.; Chen, Y.; Hu, C.; Du, G.; Liao, R.; Li, H. Selection and validation of internal reference genes for qPCR in Polygonatum cyrtonema tubers at different development stages and in response to abiotic stress. Chin. J. Chin. Mater. Med. 2020, 45, 5967–5975. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Ban, J.; Cai, R.; Zhang, X.; Lai, C.; Chen, Y.; Li, X.; Chen, C.; Chen, Y.; Zhang, Z.; et al. Metabolic Composition and Quality Traits of Polygonatum cyrtonema Hua from Different Germplasms and Age Sections Based on Widely Targeted Metabolomics Analysis. Int. J. Mol. Sci. 2023, 24, 6077. https://doi.org/10.3390/ijms24076077
Wang Q, Ban J, Cai R, Zhang X, Lai C, Chen Y, Li X, Chen C, Chen Y, Zhang Z, et al. Metabolic Composition and Quality Traits of Polygonatum cyrtonema Hua from Different Germplasms and Age Sections Based on Widely Targeted Metabolomics Analysis. International Journal of Molecular Sciences. 2023; 24(7):6077. https://doi.org/10.3390/ijms24076077
Chicago/Turabian StyleWang, Qingshuang, Jingjie Ban, Roudi Cai, Xueying Zhang, Chunwang Lai, Yan Chen, Xiaoli Li, Cuirong Chen, Yukun Chen, Zihao Zhang, and et al. 2023. "Metabolic Composition and Quality Traits of Polygonatum cyrtonema Hua from Different Germplasms and Age Sections Based on Widely Targeted Metabolomics Analysis" International Journal of Molecular Sciences 24, no. 7: 6077. https://doi.org/10.3390/ijms24076077
APA StyleWang, Q., Ban, J., Cai, R., Zhang, X., Lai, C., Chen, Y., Li, X., Chen, C., Chen, Y., Zhang, Z., Lai, Z., & Lin, Y. (2023). Metabolic Composition and Quality Traits of Polygonatum cyrtonema Hua from Different Germplasms and Age Sections Based on Widely Targeted Metabolomics Analysis. International Journal of Molecular Sciences, 24(7), 6077. https://doi.org/10.3390/ijms24076077