Glyphosate and Aminomethylphosphonic Acid (AMPA) Modulate Glutathione S-Transferase in Non-Tumorigenic Prostate Cells
Abstract
:1. Introduction
2. Results
2.1. GLY and AMPA Alter the Viability and Proliferation of Prostate Cells
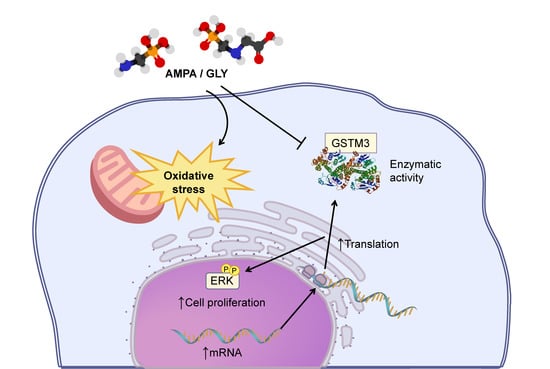
2.2. GLY and AMPA Upregulate GSTM3 Transcripts in PNT2 Cells
2.3. Expression of GSTM3 and Enzymatic Activity in PNT2 Cells
2.4. Effects of GLY and AMPA on Oxidative Stress and GAS5 Transcripts
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Chemicals
4.3. MTT
4.4. Colony Formation
4.5. qPCR Analysis
4.6. Western Blot
4.7. Enzymatic Activity
4.8. Oxyblot Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA ERS—Brazil’s Momentum as a Global Agricultural Supplier Faces Headwinds. Available online: https://ers.usda.gov/AMBER-WAVES/2022/SEPTEMBER/BRAZIL-S-MOMENTUM-AS-A-GLOBAL-AGRICULTURAL-SUPPLIER-FACES-HEADWINDS/ (accessed on 6 December 2022).
- Dekker, M. Economic Benefits of Pest Management. In Encyclopedia of Pest Management; Rajinder Peshin: New York, NY, USA, 2002; pp. 224–227. ISBN 0-618-24906-0. [Google Scholar]
- World Population Prospects-Population Division-United Nations. Available online: https://population.un.org/wpp/ (accessed on 27 December 2022).
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide Pesticide Usage and Its Impacts on Ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef] [Green Version]
- Campanale, C.; Massarelli, C.; Losacco, D.; Bisaccia, D.; Triozzi, M.; Uricchio, V.F. The Monitoring of Pesticides in Water Matrices and the Analytical Criticalities: A Review. TrAC Trends Anal. Chem. 2021, 144, 116423. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Nguyen, T.D.; Vu, M.T.; Duong, H.A.; Pham, H.V. Determination of Glyphosate, Glufosinate, and Their Major Metabolites in Tea Infusions by Dual-Channel Capillary Electrophoresis Following Solid-Phase Extraction. J. Anal. Methods Chem. 2022, 2022, 5687025. [Google Scholar] [CrossRef]
- van Bruggen, A.H.C.; Finckh, M.R.; He, M.; Ritsema, C.J.; Harkes, P.; Knuth, D.; Geissen, V. Indirect Effects of the Herbicide Glyphosate on Plant, Animal and Human Health Through Its Effects on Microbial Communities. Front. Environ. Sci. 2021, 9, 763917. [Google Scholar] [CrossRef]
- Rezende, E.C.N.; de Moraes, J.B.; Carneiro, F.M.; Guimarães, T.S.; Pires, D.d.J.; Wastowski, I.J. Glyphosate: A “Weed” in Brazilian Health. BJSTR 2021, 38, 30440–30445. [Google Scholar] [CrossRef]
- von Mérey, G.; Manson, P.S.; Mehrsheikh, A.; Sutton, P.; Levine, S.L. Glyphosate and Aminomethylphosphonic Acid Chronic Risk Assessment for Soil Biota. Environ. Toxicol. Chem. 2016, 35, 2742–2752. [Google Scholar] [CrossRef]
- Bacci, F.; Campo, P. Emerging and Less Commonly Recognized Chemical Contaminants: Organic Micropollutants. In Encyclopedia of Inland Waters, 2nd ed.; Mehner, T., Tockner, K., Eds.; Elsevier: Oxford, UK, 2022; pp. 247–259. ISBN 978-0-12-822041-2. [Google Scholar]
- Connolly, A.; Coggins, M.A.; Galea, K.S.; Jones, K.; Kenny, L.; McGowan, P.; Basinas, I. Evaluating Glyphosate Exposure Routes and Their Contribution to Total Body Burden: A Study Among Amenity Horticulturalists. Ann. Work Expo. Health 2019, 63, 133–147. [Google Scholar] [CrossRef] [Green Version]
- Peillex, C.; Pelletier, M. The Impact and Toxicity of Glyphosate and Glyphosate-Based Herbicides on Health and Immunity. J. Immunotoxicol. 2020, 17, 163–174. [Google Scholar] [CrossRef]
- Motojyuku, M.; Saito, T.; Akieda, K.; Otsuka, H.; Yamamoto, I.; Inokuchi, S. Determination of Glyphosate, Glyphosate Metabolites, and Glufosinate in Human Serum by Gas Chromatography-Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 875, 509–514. [Google Scholar] [CrossRef]
- Zouaoui, K.; Dulaurent, S.; Gaulier, J.M.; Moesch, C.; Lachâtre, G. Determination of Glyphosate and AMPA in Blood and Urine from Humans: About 13 Cases of Acute Intoxication. Forensic. Sci. Int. 2013, 226, e20–e25. [Google Scholar] [CrossRef]
- Stur, E.; Aristizabal-Pachon, A.F.; Peronni, K.C.; Agostini, L.P.; Waigel, S.; Chariker, J.; Miller, D.M.; Thomas, S.D.; Rezzoug, F.; Detogni, R.S.; et al. Glyphosate-Based Herbicides at Low Doses Affect Canonical Pathways in Estrogen Positive and Negative Breast Cancer Cell Lines. PLoS ONE 2019, 14, e0219610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- All News—ECHA. Available online: https://echa.europa.eu/-/glyphosate-not-classified-as-a-carcinogen-by-echa (accessed on 27 December 2022).
- Andreotti, G.; Koutros, S.; Hofmann, J.N.; Sandler, D.P.; Lubin, J.H.; Lynch, C.F.; Lerro, C.C.; De Roos, A.J.; Parks, C.G.; Alavanja, M.C.; et al. Glyphosate Use and Cancer Incidence in the Agricultural Health Study. J. Natl. Cancer Inst. 2018, 110, 509–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christmann, M.; Kaina, B. Transcriptional Regulation of Human DNA Repair Genes Following Genotoxic Stress: Trigger Mechanisms, Inducible Responses and Genotoxic Adaptation. Nucleic Acids Res. 2013, 41, 8403–8420. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Kaur, S.; Lata, M. Evaluation of DNA Damage in Agricultural Workers Exposed to Pesticides Using Single Cell Gel Electrophoresis (Comet) Assay. Indian J. Hum. Genet. 2011, 17, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Marino, M.; Mele, E.; Viggiano, A.; Nori, S.L.; Meccariello, R.; Santoro, A. Pleiotropic Outcomes of Glyphosate Exposure: From Organ Damage to Effects on Inflammation, Cancer, Reproduction and Development. Int. J. Mol. Sci. 2021, 22, 12606. [Google Scholar] [CrossRef]
- Woźniak, E.; Sicińska, P.; Michałowicz, J.; Woźniak, K.; Reszka, E.; Huras, B.; Zakrzewski, J.; Bukowska, B. The Mechanism of DNA Damage Induced by Roundup 360 PLUS, Glyphosate and AMPA in Human Peripheral Blood Mononuclear Cells—Genotoxic Risk Assessement. Food Chem. Toxicol. 2018, 120, 510–522. [Google Scholar] [CrossRef]
- Ojelade, B.S.; Durowoju, O.S.; Adesoye, P.O.; Gibb, S.W.; Ekosse, G.-I. Review of Glyphosate-Based Herbicide and Aminomethylphosphonic Acid (AMPA): Environmental and Health Impacts. Appl. Sci. 2022, 12, 8789. [Google Scholar] [CrossRef]
- Mesnage, R.; Ibragim, M.; Mandrioli, D.; Falcioni, L.; Tibaldi, E.; Belpoggi, F.; Brandsma, I.; Bourne, E.; Savage, E.; Mein, C.A.; et al. Comparative Toxicogenomics of Glyphosate and Roundup Herbicides by Mammalian Stem Cell-Based Genotoxicity Assays and Molecular Profiling in Sprague-Dawley Rats. Toxicol. Sci. 2022, 186, 83–101. [Google Scholar] [CrossRef]
- Franke, A.A.; Li, X.; Shvetsov, Y.B.; Lai, J.F. Pilot Study on the Urinary Excretion of the Glyphosate Metabolite Aminomethylphosphonic Acid and Breast Cancer Risk: The Multiethnic Cohort Study. Env. Pollut 2021, 277, 116848. [Google Scholar] [CrossRef]
- Li, Q.; Lambrechts, M.J.; Zhang, Q.; Liu, S.; Ge, D.; Yin, R.; Xi, M.; You, Z. Glyphosate and AMPA Inhibit Cancer Cell Growth through Inhibiting Intracellular Glycine Synthesis. Drug Des. Dev. Ther. 2013, 7, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Parajuli, K.R.; Zhang, Q.; Liu, S.; You, Z. Aminomethylphosphonic Acid and Methoxyacetic Acid Induce Apoptosis in Prostate Cancer Cells. Int. J. Mol. Sci. 2015, 16, 11750–11765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parajuli, K.R.; Zhang, Q.; Liu, S.; You, Z. Aminomethylphosphonic Acid Inhibits Growth and Metastasis of Human Prostate Cancer in an Orthotopic Xenograft Mouse Model. Oncotarget 2016, 7, 10616–10626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, S.S.; Batool, R.; Masood, N.; Yasmin, A. Risk Factors for Prostate Cancer: A Multifactorial Case-Control Study. Curr. Probl. Cancer 2018, 42, 337–343. [Google Scholar] [CrossRef]
- Brasil-Estimativa Dos Casos Novos. Available online: https://www.gov.br/inca/pt-br/assuntos/cancer/numeros/estimativa/estado-capital/brasil/brasil-consolidado (accessed on 27 December 2022).
- Matsushita, M.; Fujita, K.; Nonomura, N. Influence of Diet and Nutrition on Prostate Cancer. Int. J. Mol. Sci. 2020, 21, 1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Yang, Q.; Xu, L.; Rao, Z.; Cao, D.; Gao, M.; Liu, S. Protein Target Identification and Toxicological Mechanism Investigation of Silver Nanoparticles-Induced Hepatotoxicity by Integrating Proteomic and Metallomic Strategies. Part. Fibre Toxicol. 2019, 16, 46. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Cai, Y.; Wang, C.; Huang, M.; Chen, J. LncRNA GAS5 Regulates the Proliferation, Migration, Invasion and Apoptosis of Brain Glioma Cells through Targeting GSTM3 Expression. The Effect of LncRNA GAS5 on Glioma Cells. J. Neurooncol. 2019, 143, 525–536. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. Pesticides: An Update of Human Exposure and Toxicity. Arch. Toxicol. 2017, 91, 549–599. [Google Scholar] [CrossRef]
- Pardo, L.A.; Beane Freeman, L.E.; Lerro, C.C.; Andreotti, G.; Hofmann, J.N.; Parks, C.G.; Sandler, D.P.; Lubin, J.H.; Blair, A.; Koutros, S. Pesticide Exposure and Risk of Aggressive Prostate Cancer among Private Pesticide Applicators. Environ. Health 2020, 19, 30. [Google Scholar] [CrossRef] [Green Version]
- Toni, L.R.M.; de Santana, H.; Zaia, D.A.M. Adsorção de glifosato sobre solos e minerais. Quím. Nova 2006, 29, 829–833. [Google Scholar] [CrossRef] [Green Version]
- Alavanja, M.C.R.; Hoppin, J.A.; Kamel, F. Health Effects of Chronic Pesticide Exposure: Cancer and Neurotoxicity. Annu. Rev. Public Health 2004, 25, 155–197. [Google Scholar] [CrossRef]
- Di Donato, M.; Cernera, G.; Giovannelli, P.; Galasso, G.; Bilancio, A.; Migliaccio, A.; Castoria, G. Recent Advances on Bisphenol-A and Endocrine Disruptor Effects on Human Prostate Cancer. Mol. Cell. Endocrinol. 2017, 457, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Robitaille, C.N.; Rivest, P.; Sanderson, J.T. Antiandrogenic Mechanisms of Pesticides in Human LNCaP Prostate and H295R Adrenocortical Carcinoma Cells. Toxicol. Sci. 2015, 143, 126–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EDSP: Weight of Evidence Analysis of Potential Interaction with the Estrogen, Androgen or Thyroid Pathways. Available online: https://napavalleyregister.com/edsp-weight-of-evidence-analysis-of-potential-interaction-with-the/pdf_5ca5e688-156a-54d6-b248-d103e6591633.html (accessed on 31 January 2023).
- Authority (EFSA), E.F.S. Conclusion on the Peer Review of the Pesticide Risk Assessment of the Active Substance Glyphosate. EFSA J. 2015, 13, 4302. [Google Scholar] [CrossRef]
- Abdel-Halim, K.Y.; Osman, S.R. Cytotoxicity and Oxidative Stress Responses of Imidacloprid and Glyphosate in Human Prostate Epithelial WPM-Y.1 Cell Line. J. Toxicol. 2020, 2020, 4364650. [Google Scholar] [CrossRef] [PubMed]
- Silva-Oliveira, R.; Pereira, F.F.; Petronilho, S.; Martins, A.T.; Lameirinhas, A.; Constâncio, V.; Caldas-Ribeiro, I.; Salta, S.; Lopes, P.; Antunes, L.; et al. Clinical Significance of ARID1A and ANXA1 in HER-2 Positive Breast Cancer. J. Clin. Med. 2020, 9, 3911. [Google Scholar] [CrossRef]
- Smitherman, A.B.; Mohler, J.L.; Maygarden, S.J.; Ornstein, D.K. Expression of Annexin I, II and VII Proteins in Androgen Stimulated and Recurrent Prostate Cancer. J. Urol. 2004, 171, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Mota, S.T.S.; Vecchi, L.; Alves, D.A.; Cordeiro, A.O.; Guimarães, G.S.; Campos-Fernández, E.; Maia, Y.C.P.; Dornelas, B.d.C.; Bezerra, S.M.; de Andrade, V.P.; et al. Annexin A1 Promotes the Nuclear Localization of the Epidermal Growth Factor Receptor in Castration-Resistant Prostate Cancer. Int. J. Biochem. Cell Biol. 2020, 127, 105838. [Google Scholar] [CrossRef]
- Yang, W.; Wang, K.; Ma, J.; Hui, K.; Lv, W.; Ma, Z.; Huan, M.; Luo, L.; Wang, X.; Li, L.; et al. Inhibition of Androgen Receptor Signaling Promotes Prostate Cancer Cell Migration via Upregulation of Annexin A1 Expression. Arch. Med. Res. 2021, 52, 174–181. [Google Scholar] [CrossRef]
- Inokuchi, J.; Lau, A.; Tyson, D.R.; Ornstein, D.K. Loss of annexin A1 disrupts normal prostate glandular structure by inducing autocrine IL-6 signaling. Carcinogenesis 2009, 30, 1082–1088. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Kyprianou, N. Mechanisms Navigating the TGF-β Pathway in Prostate Cancer. Asian J. Urol. 2015, 2, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Yang, J.; You, L.; Dai, M.; Zhao, Y. GSTM3 Function and Polymorphism in Cancer: Emerging but Promising. Cancer Manag. Res. 2020, 12, 10377–10388. [Google Scholar] [CrossRef] [PubMed]
- Checa-Rojas, A.; Delgadillo-Silva, L.F.; Velasco-Herrera, M.d.C.; Andrade-Domínguez, A.; Gil, J.; Santillán, O.; Lozano, L.; Toledo-Leyva, A.; Ramírez-Torres, A.; Talamas-Rohana, P.; et al. GSTM3 and GSTP1: Novel Players Driving Tumor Progression in Cervical Cancer. Oncotarget 2018, 9, 21696–21714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medeiros, R.; Vasconcelos, A.; Costa, S.; Pinto, D.; Ferreira, P.; Lobo, F.; Morais, A.; Oliveira, J.; Lopes, C. Metabolic Susceptibility Genes and Prostate Cancer Risk in a Southern European Population: The Role of Glutathione S-Transferases GSTM1, GSTM3, and GSTT1 Genetic Polymorphisms. Prostate 2004, 58, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, W.; Jiang, J.; Bao, E.; Xu, D.; Zeng, Y.; Tao, L.; Qiu, J. Downregulation of GAS5 Promotes Bladder Cancer Cell Proliferation, Partly by Regulating CDK6. PLoS ONE 2013, 8, e73991. [Google Scholar] [CrossRef]
- Tao, R.; Hu, S.; Wang, S.; Zhou, X.; Zhang, Q.; Wang, C.; Zhao, X.; Zhou, W.; Zhang, S.; Li, C.; et al. Association between Indel Polymorphism in the Promoter Region of LncRNA GAS5 and the Risk of Hepatocellular Carcinoma. Carcinogenesis 2015, 36, 1136–1143. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Jin, F.; Xia, R.; Kong, R.; Li, J.; Xu, T.; Liu, Y.; Zhang, E.; Liu, X.; De, W. Decreased Expression of Long Noncoding RNA GAS5 Indicates a Poor Prognosis and Promotes Cell Proliferation in Gastric Cancer. BMC Cancer 2014, 14, 319. [Google Scholar] [CrossRef] [Green Version]
- Qiao, H.-P.; Gao, W.-S.; Huo, J.-X.; Yang, Z.-S. Long Non-Coding RNA GAS5 Functions as a Tumor Suppressor in Renal Cell Carcinoma. Asian Pac. J. Cancer Prev. 2013, 14, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Liu, W.; Li, F.; Zhao, W.; Qin, C. Decreased Expression of LncRNA GAS5 Predicts a Poor Prognosis in Cervical Cancer. Int J. Clin. Exp. Pathol 2014, 7, 6776–6783. [Google Scholar]
- Pickard, M.R.; Mourtada-Maarabouni, M.; Williams, G.T. Long Non-Coding RNA GAS5 Regulates Apoptosis in Prostate Cancer Cell Lines. Biochim. Biophys. Acta 2013, 1832, 1613–1623. [Google Scholar] [CrossRef] [Green Version]
- Woźniak, E.; Reszka, E.; Jabłońska, E.; Michałowicz, J.; Huras, B.; Bukowska, B. Glyphosate and AMPA Induce Alterations in Expression of Genes Involved in Chromatin Architecture in Human Peripheral Blood Mononuclear Cells (In Vitro). Int. J. Mol. Sci. 2021, 22, 2966. [Google Scholar] [CrossRef]
- Lemke, N.; Murawski, A.; Schmied-Tobies, M.I.H.; Rucic, E.; Hoppe, H.-W.; Conrad, A.; Kolossa-Gehring, M. Glyphosate and Aminomethylphosphonic Acid (AMPA) in Urine of Children and Adolescents in Germany—Human Biomonitoring Results of the German Environmental Survey 2014–2017 (GerES V). Environ. Int. 2021, 156, 106769. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, J.; Lou, N.; Lu, W.; Xu, J.; Jiang, H.; Ye, G. Long Noncoding RNA GAS5 Inhibits Proliferation and Metastasis in Papillary Thyroid Carcinoma through the IFN/STAT1 Signaling Pathway. Pathol. Res. Pract. 2022, 233, 153856. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases. The First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]




| Cell Lineages–IC50 (mM) | ||||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | |||||
| Herbicides | PNT2 | LNCaP | PC-3 | PNT2 | LNCaP | PC-3 |
| GLY | 15.15 | 13.04 | 37.59 | 11.54 | 10.48 | 22.85 |
| AMPA | ND | 30.54 | ND | 24.68 | 19.15 | ND |
| Gene | Treatment (mM) | RNA Expression | |
|---|---|---|---|
| Fold Change | |||
| PNT2 | p Value | ||
| ANXA1 | Gly 5 | 1.34 | ns |
| Gly 10 | 2.05 | *** | |
| AMPA 10 | 2.23 | *** | |
| AMPA 20 | 3.97 | **** | |
| CDH1 | Gly 5 | 1.57 | *** |
| Gly 10 | 1.46 | ** | |
| AMPA 10 | 1.42 | ** | |
| AMPA 20 | 1.12 | ns | |
| GSTM3 | Gly 5 | 8.92 | **** |
| Gly 10 | 7.85 | **** | |
| AMPA 10 | 17.51 | **** | |
| AMPA 20 | 34.86 | **** | |
| IL6 | Gly 5 | −1.16 | ns |
| Gly 10 | −2.54 | **** | |
| AMPA 10 | 1.11 | ns | |
| AMPA 20 | −3.02 | **** | |
| TGFβ1 | Gly 5 | 1.29 | ns |
| Gly 10 | 1.74 | **** | |
| AMPA 10 | 2.05 | **** | |
| AMPA 20 | 2.30 | **** | |
| VIM | Gly 5 | # | # |
| Gly 10 | # | # | |
| AMPA 10 | # | # | |
| AMPA 20 | # | # | |
 . # Vimentin was not expressed in PNT2 cells. ** p < 0.01; *** p < 0.001 and **** p < 0.0001. ns: non-significant. Annexin A1 (ANXA1), cadherin 1 (CDH1), glutathione S-transferase mu 3 (GSTM3), interleukin 6 (IL6), transforming growth factor beta 1 (TGFβ1), and vimentin (VIM).
. # Vimentin was not expressed in PNT2 cells. ** p < 0.01; *** p < 0.001 and **** p < 0.0001. ns: non-significant. Annexin A1 (ANXA1), cadherin 1 (CDH1), glutathione S-transferase mu 3 (GSTM3), interleukin 6 (IL6), transforming growth factor beta 1 (TGFβ1), and vimentin (VIM).| Gene | Sequence (5′-3′) | Amplicon (pb) |
|---|---|---|
| ANXA1 | F: GATTCAGATGCCAGGGCCT R: CACTCTGCGAAGTTGTGGAT | 110 |
| B2M | F: CCTGCCGTGTGAACCATGT R: ACTGGGATATTCGTGGGCTG | 94 |
| CDH1 | F: GTCATTGAGCCTGGCAATTTAG R: GTTGAGACTCCTCCATTCCTTC | 97 |
| GAS5 | F: CTTGCCTGGACCAGCTTAAT R: CAAGCCGACTCTCCATACCT | 82 |
| GSTM3 | F: ACTGGGATATTCGTGGGCTG R: CGCAAGATGGCATTGCTCT | 214 |
| IL6 | F: GATTCCAAAGATGTAGCCGCC R: ATTTTCACCAGGCAAGTCTCCTC | 242 |
| TGF-β1 | F: GTACCTGAACCCGTGTTGCTC R: CAGGAATTGTTGCTGTATTTCTGG | 108 |
| VIM | F: ACTAGAGATGGACAGGTTATCA R: GTAGGAGTGTCGGTTGTTAAG | 218 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, D.S.; Vecchi, L.; Barros, D.C.T.; Arruda, V.M.; Ferreira, H.S.V.; da Silva, M.F.; Guerra, J.F.d.C.; Siqueira, R.P.; Araújo, T.G. Glyphosate and Aminomethylphosphonic Acid (AMPA) Modulate Glutathione S-Transferase in Non-Tumorigenic Prostate Cells. Int. J. Mol. Sci. 2023, 24, 6323. https://doi.org/10.3390/ijms24076323
Borges DS, Vecchi L, Barros DCT, Arruda VM, Ferreira HSV, da Silva MF, Guerra JFdC, Siqueira RP, Araújo TG. Glyphosate and Aminomethylphosphonic Acid (AMPA) Modulate Glutathione S-Transferase in Non-Tumorigenic Prostate Cells. International Journal of Molecular Sciences. 2023; 24(7):6323. https://doi.org/10.3390/ijms24076323
Chicago/Turabian StyleBorges, Dayanne Silva, Lara Vecchi, Deysse Carla Tolentino Barros, Vinícius Marques Arruda, Helen Soares Valença Ferreira, Matheus Fernandes da Silva, Joyce Ferreira da Costa Guerra, Raoni Pais Siqueira, and Thaise Gonçalves Araújo. 2023. "Glyphosate and Aminomethylphosphonic Acid (AMPA) Modulate Glutathione S-Transferase in Non-Tumorigenic Prostate Cells" International Journal of Molecular Sciences 24, no. 7: 6323. https://doi.org/10.3390/ijms24076323
APA StyleBorges, D. S., Vecchi, L., Barros, D. C. T., Arruda, V. M., Ferreira, H. S. V., da Silva, M. F., Guerra, J. F. d. C., Siqueira, R. P., & Araújo, T. G. (2023). Glyphosate and Aminomethylphosphonic Acid (AMPA) Modulate Glutathione S-Transferase in Non-Tumorigenic Prostate Cells. International Journal of Molecular Sciences, 24(7), 6323. https://doi.org/10.3390/ijms24076323