Analyses of the Cellular Interactions between the Ossification of Collagen-Based Barrier Membranes and the Underlying Bone Defects
Abstract
:1. Introduction
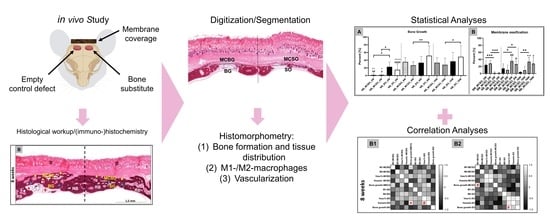
2. Results
2.1. Histological Results
2.2. Histomorphometrical Results
2.2.1. Bone Regeneration
2.2.2. Immune Response
2.2.3. Vascularization
2.3. Correlation Analyses
3. Discussion
4. Materials and Methods
4.1. Biomaterials
4.1.1. Collagen-Based Barrier Membrane
4.1.2. Xenogeneic Bone Substitute Material
4.2. In Vivo Study
4.2.1. Experimental Design
4.2.2. Histological Evaluation
4.2.3. Histological Analyses
4.2.4. Histomorphometry
- i
- Membrane compartment above bone graft-filled bone defects (MCBG);
- ii
- Membrane compartment above sham defects (MCSO);
- iii
- Bone graft-filled bone defects (BG);
- iv
- Sham bone defects (SO).
4.3. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [Green Version]
- Rider, P.; Kačarević, Z.P.; Alkildani, S.; Retnasingh, S.; Schnettler, R.; Barbeck, M. Additive Manufacturing for Guided Bone Regeneration: A Perspective for Alveolar Ridge Augmentation. Int. J. Mol. Sci. 2018, 19, 3308. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Fan, L.; Alkildani, S.; Liu, L.; Emmert, S.; Najman, S.; Rimashevskiy, D.; Schnettler, R.; Jung, O.; Xiong, X.; et al. Barrier Membranes for Guided Bone Regeneration (GBR): A Focus on Recent Advances in Collagen Membranes. Int. J. Mol. Sci. 2022, 23, 14987. [Google Scholar] [CrossRef] [PubMed]
- Kapogianni, E.; Alkildani, S.; Radenkovic, M.; Xiong, X.; Krastev, R.; Stöwe, I.; Bielenstein, J.; Jung, O.; Najman, S.; Barbeck, M.; et al. The Early Fragmentation of a Bovine Dermis-Derived Collagen Barrier Membrane Contributes to Transmembraneous Vascularization—A Possible Paradigm Shift for Guided Bone Regeneration. Membranes 2021, 11, 185. [Google Scholar] [CrossRef]
- Barbeck, M.; Lorenz, J.; Kubesch, A.; Böhm, N.; Booms, P.; Choukroun, J.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Porcine Dermis-Derived Collagen Membranes Induce Implantation Bed Vascularization Via Multinucleated Giant Cells: A Physiological Reaction? J. Oral Implant. 2015, 41, e238–e251. [Google Scholar] [CrossRef] [PubMed]
- Jung, O.; Radenkovic, M.; Stojanović, S.; Lindner, C.; Batinic, M.; Görke, O.; Pissarek, J.; Pröhl, A.; Najman, S.; Barbeck, M. In Vitro and In Vivo Biocompatibility Analysis of a New Transparent Collagen-based Wound Membrane for Tissue Regeneration in Different Clinical Indications. In Vivo 2020, 34, 2287–2295. [Google Scholar] [CrossRef]
- Radenković, M.; Alkildani, S.; Stoewe, I.; Bielenstein, J.; Sundag, B.; Bellmann, O.; Jung, O.; Najman, S.; Stojanović, S.; Barbeck, M. Comparative In Vivo Analysis of the Integration Behavior and Immune Response of Collagen-Based Dental Barrier Mem-branes for Guided Bone Regeneration (GBR). Membranes 2021, 11, 712. [Google Scholar] [CrossRef]
- Lee, J.; Byun, H.; Perikamana, S.K.M.; Lee, S.; Shin, H. Current Advances in Immunomodulatory Biomaterials for Bone Regeneration. Adv. Health Mater. 2019, 8, e1801106. [Google Scholar] [CrossRef]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [Green Version]
- Delavary, B.M.; van der Veer, W.M.; van Egmond, M.; Niessen, F.B.; Beelen, R.H. Macrophages in skin injury and repair. Immunobiology 2011, 216, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef]
- Rothamel, D.; Schwarz, F.; Fienitz, T.; Smeets, R.; Dreiseidler, T.; Ritter, L.; Happe, A.; Zoller, J. Biocompatibility and biodeg-radation of a native porcine pericardium membrane: Results of in vitro and in vivo examinations. Int. J. Oral Maxillofac. Implant. 2012, 27, 146–154. [Google Scholar]
- Barbeck, M.; Lorenz, J.; Holthaus, M.G.; Raetscho, N.; Kubesch, A.; Booms, P.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Porcine Dermis and Pericardium-Based, Non–Cross-Linked Materials Induce Multinucleated Giant Cells After Their In Vivo Implantation: A Physiological Reaction? J. Oral Implant. 2015, 41, e267–e281. [Google Scholar] [CrossRef] [PubMed]
- Dau, M.; Volprich, L.; Grambow, E.; Vollmar, B.; Frerich, B.; Al-Nawas, B.; Kämmerer, P.W. Collagen membranes of dermal and pericardial origin—In vivo evolvement of vascularization over time. J. Biomed. Mater. Res. Part A 2020, 108, 2368–2378. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S. Non-cross-linked porcine-based collagen I–III membranes do not require high vascularization rates for their integration within the implantation bed: A paradigm shift. Acta Biomater. 2012, 8, 3061–3072. [Google Scholar] [CrossRef]
- Pröhl, A.; Batinic, M.; Alkildani, S.; Hahn, M.; Radenkovic, M.; Najman, S.; Jung, O.; Barbeck, M. In Vivo Analysis of the Biocompatibility and Bone Healing Capacity of a Novel Bone Grafting Material Combined with Hyaluronic Acid. Int. J. Mol. Sci. 2021, 22, 4818. [Google Scholar] [CrossRef]
- Stöwe, I.; Pissarek, J.; Moosmann, P.; Pröhl, A.; Pantermehl, S.; Bielenstein, J.; Radenkovic, M.; Jung, O.; Najman, S.; Alkildani, S.; et al. Ex Vivo and In Vivo Analysis of a Novel Porcine Aortic Patch for Vascular Reconstruction. Int. J. Mol. Sci. 2021, 22, 7623. [Google Scholar] [CrossRef]
- Tawil, G.; Barbeck, M.; Unger, R.; Tawil, P.; Witte, F. Sinus Floor Elevation Using the Lateral Approach and Window Repositioning and a Xenogeneic Bone Substitute as a Grafting Material: A Histologic, Histomorphometric, and Radiographic Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 1089–1096. [Google Scholar] [CrossRef]
- Klein, M.O.; Kämmerer, P.W.; Götz, H.; Duschner, H.; Wagner, W. Long-Term Bony Integration and Resorption Kinetics of a Xenogeneic Bone Substitute After Sinus Floor Augmentation: Histomorphometric Analyses of Human Biopsy Specimens. Int. J. Periodontics Restor. Dent. 2013, 33, e101–e110. [Google Scholar] [CrossRef]
- Bielenstein, J.; Radenković, M.; Najman, S.; Liu, L.; Ren, Y.; Cai, B.; Beuer, F.; Rimashevskiy, D.; Schnettler, R.; Alkildani, S.; et al. In Vivo Analysis of the Regeneration Capacity and Immune Response to Xenogeneic and Synthetic Bone Substitute Materials. Int. J. Mol. Sci. 2022, 23, 10636. [Google Scholar] [CrossRef]
- Ghayor, C.; Weber, F.E. Osteoconductive Microarchitecture of Bone Substitutes for Bone Regeneration Revisited. Front. Physiol. 2018, 9, 960. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Udeabor, S.E.; Lorenz, J.; Kubesch, A.; Choukroun, J.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. Induction of multinucleated giant cells in response to small sized bovine bone substitute (Bio-OssTM) results in an enhanced early implantation bed vascularization. Ann. Maxillofac. Surg. 2014, 4, 150–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zubery, Y.; Goldlust, A.; Alves, A.; Nir, E. Ossification of a Novel Cross-Linked Porcine Collagen Barrier in Guided Bone Regeneration in Dogs. J. Periodontol. 2007, 78, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Zubery, Y.; Nir, E.; Goldlust, A. Ossification of a Collagen Membrane Cross-Linked by Sugar: A Human Case Series. J. Periodontol. 2008, 79, 1101–1107. [Google Scholar] [CrossRef]
- Taguchi, Y.; Amizuka, N.; Nakadate, M.; Ohnishi, H.; Fujii, N.; Oda, K.; Nomura, S.; Maeda, T. A histological evaluation for guided bone regeneration induced by a collagenous membrane. Biomaterials 2005, 26, 6158–6166. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Liu, R.; Chen, S.; Liu, Q.; Cai, H.; Lin, Y.; Chen, Z.; Chen, Z. Tuning the immune reaction to manipulate the cell-mediated degradation of a collagen barrier membrane. Acta Biomater. 2020, 109, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Caballe-Serrano, J.; Munar-Frau, A.; Ortiz-Puigpelat, O.; Soto-Penaloza, D.; Penarrocha, M.; Hernandez-Alfaro, F. On the search of the ideal barrier membrane for guided bone regeneration. J. Clin. Exp. Dent. 2018, 10, e477–e483. [Google Scholar] [CrossRef]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of Collagen Membranes for Bone Regeneration: A Literature Review. Materials 2020, 13, 786. [Google Scholar] [CrossRef] [Green Version]
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier membranes: More than the barrier effect? J. Clin. Periodontol. 2019, 46 (Suppl. S21), 103–123. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, J.; Akhavan, N.; Mullins, A.; Arjmandi, B. Macrophage Polarization and Osteoporosis: A Review. Nutrients 2020, 12, 2999. [Google Scholar] [CrossRef]
- Olmsted-Davis, E.; Mejia, J.; Salisbury, E.; Gugala, Z.; Davis, A.R. A Population of M2 Macrophages Associated with Bone Formation. Front. Immunol. 2021, 12, 686769. [Google Scholar] [CrossRef] [PubMed]
- Lindner, C.; Pröhl, A.; Abels, M.; Löffler, T.; Batinic, M.; Jung, O.; Barbeck, M. Specialized Histological and Histomorphometrical Analytical Methods for Biocompatibility Testing of Biomaterials for Maxillofacial Surgery in (Pre-) Clinical Studies. In Vivo 2020, 34, 3137–3152. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Booms, P.; Unger, R.; Hoffmann, V.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Multinucleated giant cells in the implant bed of bone substitutes are foreign body giant cells-New insights into the material-mediated healing process. J. Biomed. Mater. Res. Part A 2017, 105, 1105–1111. [Google Scholar] [CrossRef]
- Sridharan, R.; Cameron, A.R.; Kelly, D.J.; Kearney, C.J.; O’Brien, F.J. Biomaterial based modulation of macrophage polarization: A review and suggested design principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Barbeck, M.; Dard, M.; Kokkinopoulou, M.; Markl, J.; Booms, P.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. Small-sized granules of biphasic bone substitutes support fast implant bed vascularization. Biomatter 2015, 5, e1056943. [Google Scholar] [CrossRef] [Green Version]
- Zarubova, J.; Hasani-Sadrabadi, M.M.; Ardehali, R.; Li, S. Immunoengineering strategies to enhance vascularization and tissue regeneration. Adv. Drug Deliv. Rev. 2022, 184, 114233. [Google Scholar] [CrossRef]
- Park, J.E.; Keller, G.A.; Ferrara, N. The vascular endothelial growth factor (VEGF) isoforms: Differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol. Biol. Cell 1993, 4, 1317–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nillesen, S.T.; Geutjes, P.J.; Wismans, R.; Schalkwijk, J.; Daamen, W.F.; van Kuppevelt, T.H. Increased angiogenesis and blood vessel maturation in acellular collagen–heparin scaffolds containing both FGF2 and VEGF. Biomaterials 2007, 28, 1123–1131. [Google Scholar] [CrossRef]
- Greenberg, J.I.; Shields, D.J.; Barillas, S.G.; Acevedo, L.M.; Murphy, E.; Huang, J.; Scheppke, L.; Stockmann, C.; Johnson, R.S.; Angle, N.; et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature 2008, 456, 809–813. [Google Scholar] [CrossRef]
- Furumatsu, T.; Shen, Z.N.; Kawai, A.; Nishida, K.; Manabe, H.; Oohashi, T.; Inoue, H.; Ninomiya, Y. Vascular endothelial growth factor principally acts as the main angiogenic factor in the early stage of human osteoblastogenesis. J. Biochem. 2003, 133, 633–639. [Google Scholar] [CrossRef]
- Grosso, A.; Burger, M.G.; Lunger, A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. It Takes Two to Tango: Coupling of Angiogenesis and Osteogenesis for Bone Regeneration. Front. Bioeng. Biotechnol. 2017, 5, 68. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.J.; West, J.L. Vascularization of engineered tissues: Approaches to promote angio-genesis in biomaterials. Curr Top Med Chem. 2008, 8, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Ratner, B.D.; Goodman, S.B.; Amar, S.; Badylak, S.F. Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 2012, 33, 3792–3802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abaricia, J.O.; Farzad, N.; Heath, T.J.; Simmons, J.; Morandini, L.; Olivares-Navarrete, R. Control of innate immune response by biomaterial surface topography, energy, and stiffness. Acta Biomater. 2021, 133, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef]
- Abels, M.; Alkildani, S.; Pröhl, A.; Xiong, X.; Krastev, R.; Korzinskas, T.; Stojanovic, S.; Jung, O.; Najman, S.; Barbeck, M. The Granule Size Mediates the In Vivo Foreign Body Response and the Integration Behavior of Bone Substitutes. Materials 2021, 14, 7372. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Huang, C.-C.; Lu, Y.; Shirazi, S.; Gajendrareddy, P.; Ravindran, S.; Cooper, L.F. Bone regeneration is mediated by macrophage extracellular vesicles. Bone 2020, 141, 115627. [Google Scholar] [CrossRef] [PubMed]
- Place, T.L.; Domann, F.E.; Case, A.J. Limitations of oxygen delivery to cells in culture: An underappreciated problem in basic and translational research. Free. Radic. Biol. Med. 2017, 113, 311–322. [Google Scholar] [CrossRef]
- Rouwkema, J.; Koopman, B.F.; van Blitterswijk, C.; Dhert, W.; Malda, J. Supply of Nutrients to Cells in Engineered Tissues. Biotechnol. Genet. Eng. Rev. 2009, 26, 163–178. [Google Scholar] [CrossRef] [Green Version]
- Wu, D. Signaling mechanisms for regulation of chemotaxis. Cell Res. 2005, 15, 52–56. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.K. Molecular regulation of vessel maturation. Nat. Med. 2003, 9, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Lobov, I.B.; Renard, R.A.; Papadopoulos, N.; Gale, N.W.; Thurston, G.; Yancopoulos, G.D.; Wiegand, S.J. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc. Natl. Acad. Sci. USA 2007, 104, 3219–3224. [Google Scholar] [CrossRef] [Green Version]
- Hellberg, C.; Östman, A.; Heldin, C.-H. PDGF and Vessel Maturation. Recent Results Cancer Res. 2010, 180, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, C.H.F.; Jung, R.E. Bone augmentation by means of barrier membranes. Periodontology 2000 2003, 33, 36–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merli, M.; Moscatelli, M.; Mariotti, G.; Pagliaro, U.; Breschi, L.; Mazzoni, A.; Nieri, M. Membranes and Bone Substitutes in a One-Stage Procedure for Horizontal Bone Augmentation: A Histologic Double-Blind Parallel Randomized Controlled Trial. Int. J. Periodontics Restor. Dent. 2015, 35, 463–471. [Google Scholar] [CrossRef] [Green Version]
- Ortolani, E.; Quadrini, F.; Bellisario, D.; Santo, L.; Polimeni, A.; Santarsiero, A. Mechanical qualification of collagen membranes used in dentistry. Ann. dell’Istituto Super. Sanita 2015, 51, 229–235. [Google Scholar] [CrossRef]
- Biomaterials, B. Jason Membrane. Available online: https://www.botiss-dental.com/pdf/botiss_membranes_EN.pdf (accessed on 1 June 2021).
- Perić Kačarević, Z.; Kavehei, F.; Houshmand, A.; Franke, J.; Smeets, R.; Rimashevskiy, D.; Wenisch, S.; Schnettler, R.; Jung, O.; Barbeck, M. Purification processes of xenogeneic bone substitutes and their impact on tissue reactions and regeneration. Int. J. Artif. Organs 2018, 41, 789–800. [Google Scholar] [CrossRef]
- Trajkovski, B.; Jaunich, M.; Müller, W.-D.; Beuer, F.; Zafiropoulos, G.-G.; Houshmand, A. Hydrophilicity, Viscoelastic, and Physicochemical Properties Variations in Dental Bone Grafting Substitutes. Materials 2018, 11, 215. [Google Scholar] [CrossRef] [Green Version]
- DIN EN ISO 10993-6; Biological Evaluation of Medical Devices—Part 6: Tests for Local Effects after Implantation. DIN Deutsches Institut für Normung: Berlin, Germany, 2017.
- Lindner, C.; Alkildani, S.; Stojanovic, S.; Najman, S.; Jung, O.; Barbeck, M. In Vivo Biocompatibility Analysis of a Novel Barrier Membrane Based on Bovine Dermis-Derived Collagen for Guided Bone Regeneration (GBR). Membranes 2022, 12, 378. [Google Scholar] [CrossRef]







| Tissue Fraction | MCBG | MCSO | BG | SO | |
|---|---|---|---|---|---|
| 2 weeks | Bone tissue (%) | 1.6 ± 3.7 | 1.6 ± 2.1 | 22.8 ± 12.5 | 15.0 ± 15.3 |
| Biomaterial (%) | 24.7 ± 12.7 | 28.6 ± 8.2 | 10.3 ± 7.5 | - | |
| 8 weeks | Bone tissue (%) | 35.5 ± 10.9 | 26.6 ± 9.4 | 33.4 ± 16.1 | 51.6 ± 25.7 |
| Biomaterial (%) | 14.5 ± 13.1 | 9.9 ± 13.9 | 19.3 ± 9.2 | - | |
| 16 weeks | Bone tissue (%) | 33.4 ± 12.7 | 27.9 ± 17.3 | 34.0 ± 23.4 | 48.8 ± 28.6 |
| Biomaterial (%) | 4.2 ± 2.1 | 9.1 ± 11.6 | 15.2 ± 10.4 | - |
| MCBG | MCSO | BG | SO | |
|---|---|---|---|---|
| CD163 (cells/mm2) | ||||
| 2 weeks | 1201 ± 571.9 | 1006 ± 562.6 | 308.7 ± 146.9 | 371.8 ± 244.3 |
| 8 weeks | 1100 ± 474.9 | 823.5 ± 413.8 | 365.3 ± 173.8 | 346.5 ± 189.8 |
| 16 weeks | 691.5 ± 320.8 | 987.8 ± 558.2 | 241.1 ± 122.0 | 297.8 ± 110.1 |
| CD11c (cells/mm2) | ||||
| 2 weeks | 503.7 ± 126.8 | 422.6 ± 170.4 | 8.8 ± 10.0 | 7.6 ± 4.6 |
| 8 weeks | 290.5 ± 190.6 | 212.4 ± 115.9 | 16.6 ± 11.8 | 20.0 ± 11.2 |
| 16 weeks | 104.2 ± 47.0 | 129.5 ± 39.4 | 14.6 ± 11.5 | 11.51 ± 5.7 |
| MCBG | MCSO | BG | SO | |
|---|---|---|---|---|
| Vessel Density (vessels/mm2) | ||||
| 2 weeks | 291.2 ± 49.4 | 253.9 ± 42.9 | 323.8 ± 73.5 | 315.0 ± 48.8 |
| 8 weeks | 184.8 ± 105.7 | 161.2 ± 80.7 | 249.7 ± 96.5 | 246.1 ± 55.8 |
| 16 weeks | 202.8 ± 82.2 | 281.8 ± 83.4 | 183.1 ± 40.8 | 259.5 ± 70.7 |
| Vascularization Percentage (%) | ||||
| 2 weeks | 5.2 ± 1.9 | 4.1 ± 1.0 | 7.2 ± 2.5 | 5.6 ± 1.6 |
| 8 weeks | 2.2 ± 1.7 | 1.4 ± 0.8 | 4.6 ± 2.4 | 2.7 ± 1.1 |
| 16 weeks | 1.9 ± 0.9 | 2.9 ± 1.4 | 2.6 ± 1.4 | 3.7 ± 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkildani, S.; Ren, Y.; Liu, L.; Rimashevskiy, D.; Schnettler, R.; Radenković, M.; Najman, S.; Stojanović, S.; Jung, O.; Barbeck, M. Analyses of the Cellular Interactions between the Ossification of Collagen-Based Barrier Membranes and the Underlying Bone Defects. Int. J. Mol. Sci. 2023, 24, 6833. https://doi.org/10.3390/ijms24076833
Alkildani S, Ren Y, Liu L, Rimashevskiy D, Schnettler R, Radenković M, Najman S, Stojanović S, Jung O, Barbeck M. Analyses of the Cellular Interactions between the Ossification of Collagen-Based Barrier Membranes and the Underlying Bone Defects. International Journal of Molecular Sciences. 2023; 24(7):6833. https://doi.org/10.3390/ijms24076833
Chicago/Turabian StyleAlkildani, Said, Yanru Ren, Luo Liu, Denis Rimashevskiy, Reinhard Schnettler, Milena Radenković, Stevo Najman, Sanja Stojanović, Ole Jung, and Mike Barbeck. 2023. "Analyses of the Cellular Interactions between the Ossification of Collagen-Based Barrier Membranes and the Underlying Bone Defects" International Journal of Molecular Sciences 24, no. 7: 6833. https://doi.org/10.3390/ijms24076833
APA StyleAlkildani, S., Ren, Y., Liu, L., Rimashevskiy, D., Schnettler, R., Radenković, M., Najman, S., Stojanović, S., Jung, O., & Barbeck, M. (2023). Analyses of the Cellular Interactions between the Ossification of Collagen-Based Barrier Membranes and the Underlying Bone Defects. International Journal of Molecular Sciences, 24(7), 6833. https://doi.org/10.3390/ijms24076833