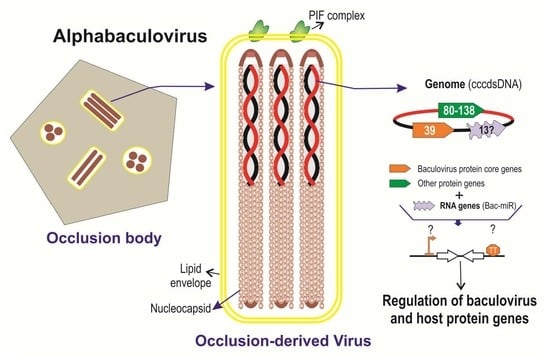
A Comprehensive Study of MicroRNA in Baculoviruses
Abstract
:1. Baculovirus Biology
2. miRNA Biogenesis and Function
3. Baculovirus-Encoded miRNAs
| Bac-miR | Virus | Host | Locus | Viral Target | Host Target | Reference |
|---|---|---|---|---|---|---|
| bmnpv-miR-1 | BmNPV | Bombycidae | Cathepsin (AB) c | bro-I (ABD), fusolin (ABG), DNA polymerase (ABGD) | GTP-binding nuclear protein Ran, serine protease | [44,45] |
| bmnpv-miR-2 | BmNPV | Bombycidae | Chitinase (AB) c | Chitinase (AB) | Eukaryotic translation initiation factor 4E-binding protein, Bombyx mori Hemolin | [44] |
| bmnpv-miR-3 | BmNPV | Bombycidae | lef5 (ABGD) c | bro-III (AB), p6.9 (ABGD), p40 (ABGD), p95 (ABGD), vlf-1 (ABGD), Fusolin (ABG), chitinase (AB) | Prophenoloxidase | [44,46] |
| bmnpv-miR-4 | BmNPV | Bombycidae | vp80 (A) | lef-8 (ABGD), p25 (A+B)C, helicase (ABGD), ORF3(AB) | Chymotrypsin-like serine protease, cadherin-like membrane protein, DEAD box polypeptide | [44] |
| BmNPV-miR-415 | BmNPV | Bombycidae | odv-e66 (AB) | - | Rapamycin isoform 2 (ToR2) | [47] |
| AcMNPV-miR-1 | AcMNPV | Noctuidae | odv-e25 (ABGD) c | ac18, odv-e25 (ABGD), ac95 (ABGD), ac15 (AB), ac131 (ABG), ac82 (AB), ac34(AB), ac101 (ABGD), ac55(AB), ac135 (AB), ac30, ac66 (ABGD), ac11(A), ac64 (AB) | - | [48,49] |
| AcMNPV-miR-2 | AcMNPV | Noctuidae | lef-6 (AB) c | lef-6 (AB), lef-11 (ABG), orf-49 (A), orf-63, orf-38 (AB) | - | [17] |
| AcMNPV-miR-3 | AcMNPV | Noctuidae | odv-C42 (ABGD) c | ac10 (AB), f-protein (ABD), ac25 (ABG), ac86, ac98 (ABGD), ac101 (ABGD), chitinase (AB) | - | [50] |
| AcMNPV-miR-4 | AcMNPV | Noctuidae | cg30 (AB) | - | Alg-2, rop | [17] |
| AcMNPV-miR-5 | AcMNPV | Noctuidae | 49k (ABGD) | Twenty predicted candidate targets without evident regulation; further experimental investigation is required | [17] | |
| agmnpv-miR-4 | AgMNPV | Noctuidae | p48 (ABGD) c | lef-8 (ABGD) | - | [51] |
| SpltNPV-miR-11684-3p | SpltNPV | Noctuidae | pk1 (AB)–hoar (AB) c | - | V-type proton ATPase catalytic subunit A-like (vATPaseA), replication factor C4 (repC4), NADH dehydrogenase[ubiquinone] 1 subunit C2-like (ndc2) | [52] |
| SpltNPV-miR-11698-3p | SpltNPV | Noctuidae | p6.9 (ABGD) c | - | Eukaryotic initiation factor 4A-like isoform 1 (eIF4A), prostaglandin reductase 1 (pgr1), H2A histone family member V (H2A), FAD-dependent oxidoreductase | [52] |
| Bac-miR | Sequence miRNA | Locus Pre-miRNA (Mature miRNA) | ORF Localization | Putative Promoter (Genomic Position) | Putative PolyA Signal (Genomic Position) |
|---|---|---|---|---|---|
| bmnpv-miR-1 | AAAUGGGCGGCGUACAGCUGG | c99262–99347 (c99318–99338) | 98756–99727 | CAGT(−79), TAAG(−537) | ATTAAA(+426) |
| bmnpv-miR-2 | GGGGUUUUUGUACGGCGGCCC | 98016–98105 (98020–98040) | c97049–98707 | TATA(−374), TAAG(−454) | ATTAAA(+459) |
| bmnpv-miR-3 | GAAAGCCAAACGAGGGCAGGCG | c80268–80356 (c80278–80299) | 79558–80355 | CAGT(−64) | AATAAA(+1969) |
| bmnpv-miR-4 | GGUGGAUGUGAUUGUUGACGACA | 83292–83385 (83308–83330) | 83240–85318 | TATA(−59), TAAG(−89) | ATTAAA(+58) |
| BmNPV-miR-415 | UCGAGUUGACGGCCGUGGGC | c33600–33463 (c33522–33541) | 32866–34974 | TATA(−122), TAAG(−149) | ATTAAA(+554) |
| AcMNPV-miR-1 | GCGACGACUCGGUUAAGGAA | c80100–80042 (c80045–80064) | 79971–80657 | TATA(−93), CAGT(−318) | AATAAA(+20) |
| AcMNPV-miR-2 | AACACCUGCCUGUAGGCGCGCUU | c23672–23599 (c23603–23625) | 23465–23986 | CAGT(−169), TATA(−348) | ATTAAA(+70) |
| AcMNPV-miR-3 | GCGGCGUAGGCUGCGCGGACGCU | 87727–87793 (87733–87755) | c86921–88006 | CAGT(−213) | AATAAA(+856) |
| AcMNPV-miR-4 | UUUCUGCAACCAAUAGACAGA | c75504–75450 (c75451–75471) | c74737–75531 | CAGT(−88), TATA(−129), TAAG(−316) | ATTAAA(+162) |
| AcMNPV-miR-5 | UAGACGAUGCCGUGCUCAUGAA | 123775–123866 (123783–123804) | 123632–125065 | TAAG(−161), TATA(−167) | AATAAA(+182) |
| agmnpv-miR-4 | GGUGGCCGCCACUUUGUUUACUU | c49475–49370 (c49450–49472) | 48600–49826 | TATA(−267) | AATAAA(+34) |
| SpltNPV-miR-11684-3p | GGUCGUGUCUUCCACUUCCUU | 3434–3522 (3486–3506) | 2392–3204 c3519–5714 | TATA(−39), CAGT(−143), TAAG(−220) | AATAAA(+360) |
| SpltNPV-miR-11698-3p | UCGAGCGGCGAUGGUAGAAG | 87923–88008 (87974–87993) | c87850–88104 | TATA(−190) | AATAAA(+135) |
4. Shared Characteristics among Described bac-miRs
5. Bioinformatics Tools for miRNA Studies
6. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rohrmann, G.F. Baculovirus Molecular Biology, 4th ed.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2019.
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect Pathogens as Biological Control Agents: Back to the Future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.C.; Aksular, M.; Graves, L.P.; Irons, S.L.; Possee, R.D.; King, L.A. Overview of the Baculovirus Expression System. CP Protein Sci. 2018, 91, 5.4.1–5.4.6. [Google Scholar] [CrossRef] [PubMed]
- Ono, C.; Okamoto, T.; Abe, T.; Matsuura, Y. Baculovirus as a Tool for Gene Delivery and Gene Therapy. Viruses 2018, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Targovnik, A.M.; Simonin, J.A.; Mc Callum, G.J.; Smith, I.; Cuccovia Warlet, F.U.; Nugnes, M.V.; Miranda, M.V.; Belaich, M.N. Solutions against Emerging Infectious and Noninfectious Human Diseases through the Application of Baculovirus Technologies. Appl. Microbiol. Biotechnol. 2021, 105, 8195–8226. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, Z. Advances in Molecular Biology of Baculoviruses. Curr. Issues Mol. Biol. 2020, 34, 183–214. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.L.; Herniou, E.A.; Jehle, J.A.; Theilmann, D.A.; Burand, J.P.; Becnel, J.J.; Krell, P.J.; Van Oers, M.M.; Mowery, J.D.; Bauchan, G.R.; et al. ICTV Virus Taxonomy Profile: Baculoviridae. J. Gen. Virol. 2018, 99, 1185–1186. [Google Scholar] [CrossRef] [PubMed]
- Jehle, J.A.; Blissard, G.W.; Bonning, B.C.; Cory, J.S.; Herniou, E.A.; Rohrmann, G.F.; Theilmann, D.A.; Thiem, S.M.; Vlak, J.M. On the Classification and Nomenclature of Baculoviruses: A Proposal for Revision. Arch. Virol. 2006, 151, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.L.; Herniou, E.A.; Bézier, A.; Jehle, J.A.; Burand, J.P.; Theilmann, D.A.; Krell, P.J.; Van Oers, M.M.; Nakai, M. ICTV Report Consortium ICTV Virus Taxonomy Profile: Nudiviridae. J. Gen. Virol. 2020, 101, 3–4. [Google Scholar] [CrossRef]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Davison, A.J.; Dempsey, D.M.; Dutilh, B.E.; García, M.L.; et al. Changes to Virus Taxonomy and to the International Code of Virus Classification and Nomenclature Ratified by the International Committee on Taxonomy of Viruses (2021). Arch. Virol. 2021, 166, 2633–2648. [Google Scholar] [CrossRef]
- Blissard, G.W.; Theilmann, D.A. Baculovirus Entry and Egress from Insect Cells. Annu. Rev. Virol. 2018, 5, 113–139. [Google Scholar] [CrossRef]
- Cerrudo, C.S.; Motta, L.F.; Cuccovia Warlet, F.U.; Lassalle, F.M.; Simonin, J.A.; Belaich, M.N. Protein-Gene Orthology in Baculoviridae: An Exhaustive Analysis to Redefine the Ancestrally Common Coding Sequences. Viruses 2023, 15, 1091. [Google Scholar] [CrossRef] [PubMed]
- Garavaglia, M.J.; Miele, S.A.B.; Iserte, J.A.; Belaich, M.N.; Ghiringhelli, P.D. The Ac53, Ac78, Ac101, and Ac103 Genes Are Newly Discovered Core Genes in the Family Baculoviridae. J. Virol. 2012, 86, 12069–12079. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.A.; Biswas, S.; Willis, L.G.; Harris, S.; Pritchard, C.; Van Oers, M.M.; Donly, B.C.; Erlandson, M.A.; Hegedus, D.D.; Theilmann, D.A. Autographa Californica Multiple Nucleopolyhedrovirus AC83 Is a Per Os Infectivity Factor (PIF) Protein Required for Occlusion-Derived Virus (ODV) and Budded Virus Nucleocapsid Assembly as Well as Assembly of the PIF Complex in ODV Envelopes. J. Virol. 2017, 91, e02115-16. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Qiu, L.; Li, G. Baculovirus-Encoded MicroRNAs: A Brief Overview and Future Prospects. Curr. Microbiol. 2019, 76, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.D.P.; Dos Santos, E.R.; Harrison, R.L.; Ribeiro, B.M.; Ardisson-Araújo, D.M.P. Identification and Analysis of Putative tRNA Genes in Baculovirus Genomes. Virus Res. 2022, 322, 198949. [Google Scholar] [CrossRef]
- Wang, J.; Xing, K.; Xiong, P.; Liang, H.; Zhu, M.; Zhao, J.; Yu, X.; Ning, X.; Li, R.; Wang, X. Identification of miRNAs Encoded by Autographa Californica Nucleopolyhedrovirus. J. Gen. Virol. 2021, 102, jgv001510. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Wu, C.-P.; Huang, Y.-H.; Huang, S.-P.; Lo, H.-R.; Chang, H.-S.; Lin, P.-H.; Wu, M.-C.; Chang, C.-J.; Chao, Y.-C. Identification of a High-Efficiency Baculovirus DNA Replication Origin That Functions in Insect and Mammalian Cells. J. Virol. 2014, 88, 13073–13085. [Google Scholar] [CrossRef]
- Miele, S.A.B.; Cerrudo, C.S.; Parsza, C.N.; Nugnes, M.V.; Mengual Gómez, D.L.; Belaich, M.N.; Ghiringhelli, P.D. Identification of Multiple Replication Stages and Origins in the Nucleopolyhedrovirus of Anticarsia Gemmatalis. Viruses 2019, 11, 648. [Google Scholar] [CrossRef]
- Wahl, M.C.; Will, C.L.; Lührmann, R. The Spliceosome: Design Principles of a Dynamic RNP Machine. Cell 2009, 136, 701–718. [Google Scholar] [CrossRef]
- Iwakawa, H.; Tomari, Y. Life of RISC: Formation, Action, and Degradation of RNA-Induced Silencing Complex. Mol. Cell 2022, 82, 30–43. [Google Scholar] [CrossRef]
- Hille, F.; Richter, H.; Wong, S.P.; Bratovič, M.; Ressel, S.; Charpentier, E. The Biology of CRISPR-Cas: Backward and Forward. Cell 2018, 172, 1239–1259. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Guo, J.K.; Wei, Y.; Dou, D.R.; Zarnegar, B.; Ma, Q.; Li, R.; Zhao, Y.; Liu, F.; Choudhry, H.; et al. Structural Modularity of the XIST Ribonucleoprotein Complex. Nat. Commun. 2020, 11, 6163. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.J.; Chang, H.Y. Unique Features of Long Non-Coding RNA Biogenesis and Function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, P. Key Mechanistic Principles and Considerations Concerning RNA Interference. Front. Plant Sci. 2020, 11, 1237. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Soto, A.; Chacón-Cerdas, R. RNAi Crop Protection Advances. Int. J. Mol. Sci. 2021, 22, 12148. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Aigner, A. Therapeutic siRNA: State-of-the-Art and Future Perspectives. BioDrugs 2022, 36, 549–571. [Google Scholar] [CrossRef] [PubMed]
- Torri, A.; Jaeger, J.; Pradeu, T.; Saleh, M.-C. The Origin of RNA Interference: Adaptive or Neutral Evolution? PLoS Biol. 2022, 20, e3001715. [Google Scholar] [CrossRef]
- Jungers, C.F.; Djuranovic, S. Modulation of miRISC-Mediated Gene Silencing in Eukaryotes. Front. Mol. Biosci. 2022, 9, 832916. [Google Scholar] [CrossRef]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef]
- Gregory, R.I.; Yan, K.; Amuthan, G.; Chendrimada, T.; Doratotaj, B.; Cooch, N.; Shiekhattar, R. The Microprocessor Complex Mediates the Genesis of microRNAs. Nature 2004, 432, 235–240. [Google Scholar] [CrossRef]
- Zapletal, D.; Kubicek, K.; Svoboda, P.; Stefl, R. Dicer Structure and Function: Conserved and Evolving Features. EMBO Rep. 2023, 24, e57215. [Google Scholar] [CrossRef] [PubMed]
- Yeom, K.-H.; Lee, Y.; Han, J.; Suh, M.R.; Kim, V.N. Characterization of DGCR8/Pasha, the Essential Cofactor for Drosha in Primary miRNA Processing. Nucleic Acids Res. 2006, 34, 4622–4629. [Google Scholar] [CrossRef] [PubMed]
- Bobadilla Ugarte, P.; Barendse, P.; Swarts, D.C. Argonaute Proteins Confer Immunity in All Domains of Life. Curr. Opin. Microbiol. 2023, 74, 102313. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA Sequences to Function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Salim, U.; Kumar, A.; Kulshreshtha, R.; Vivekanandan, P. Biogenesis, Characterization, and Functions of Mirtrons. WIREs RNA 2022, 13, e1680. [Google Scholar] [CrossRef] [PubMed]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An Overview of microRNAs: Biology, Functions, Therapeutics, and Analysis Methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef] [PubMed]
- Yamazawa, R.; Jiko, C.; Choi, S.; Park, I.Y.; Nakagawa, A.; Yamashita, E.; Lee, S.J. Structural Basis for Selective Binding of Export Cargoes by Exportin-5. Structure 2018, 26, 1393–1398.e2. [Google Scholar] [CrossRef] [PubMed]
- Riolo, G.; Cantara, S.; Marzocchi, C.; Ricci, C. miRNA Targets: From Prediction Tools to Experimental Validation. Methods Protoc. 2020, 4, 1. [Google Scholar] [CrossRef]
- Chipman, L.B.; Pasquinelli, A.E. miRNA Targeting: Growing beyond the Seed. Trends Genet. 2019, 35, 215–222. [Google Scholar] [CrossRef]
- Monsanto-Hearne, V.; Johnson, K.N. miRNA Modulation of Insect Virus Replication. Curr. Issues Mol. Biol. 2020, 34, 61–82. [Google Scholar] [CrossRef]
- Mengistu, A.A.; Tenkegna, T.A. The Role of miRNA in Plant–Virus Interaction: A Review. Mol. Biol. Rep. 2021, 48, 2853–2861. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef]
- Singh, J.; Singh, C.P.; Bhavani, A.; Nagaraju, J. Discovering microRNAs from Bombyx Mori Nucleopolyhedrosis Virus. Virology 2010, 407, 120–128. [Google Scholar] [CrossRef]
- Singh, C.P.; Singh, J.; Nagaraju, J. A Baculovirus-Encoded MicroRNA (miRNA) Suppresses Its Host miRNA Biogenesis by Regulating the Exportin-5 Cofactor Ran. J. Virol. 2012, 86, 7867–7879. [Google Scholar] [CrossRef]
- Singh, C.P.; Singh, J.; Nagaraju, J. Bmnpv-miR-3 Facilitates BmNPV Infection by Modulating the Expression of Viral P6.9 and Other Late Genes in Bombyx Mori. Insect Biochem. Mol. Biol. 2014, 49, 59–69. [Google Scholar] [CrossRef]
- Cao, X.; Huang, Y.; Xia, D.; Qiu, Z.; Shen, X.; Guo, X.; Zhao, Q. BmNPV-miR-415 up-Regulates the Expression of TOR2 via Bmo-miR-5738. Saudi J. Biol. Sci. 2017, 24, 1614–1619. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, J.; Deng, R.; Xiong, P.; Liang, H.; Wang, X. A MicroRNA Encoded by Autographa Californica Nucleopolyhedrovirus Regulates Expression of Viral Gene ODV-E25. J. Virol. 2013, 87, 13029–13034. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, J.; Deng, R.; Wang, X. Functional Regulation of an Autographa Californica Nucleopolyhedrovirus-Encoded MicroRNA, AcMNPV-miR-1, in Baculovirus Replication. J. Virol. 2016, 90, 6526–6537. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, J.; Deng, R.; Yu, X.; Wang, X. AcMNPV-miR-3 Is a miRNA Encoded by Autographa Californica Nucleopolyhedrovirus and Regulates the Viral Infection by Targeting Ac101. Virus Res. 2019, 267, 49–58. [Google Scholar] [CrossRef]
- Ferrelli, M.L.; García, M.L.; Romanowski, V.; Reyes, C.A. Identification of a microRNA Encoded by Anticarsia Gemmatalis Multiple Nucleopolyhedrovirus. Comput. Biol. Chem. 2020, 87, 107276. [Google Scholar] [CrossRef]
- Nayyar, N.; Kaur, I.; Malhotra, P.; Bhatnagar, R.K. Quantitative Proteomics of Sf21 Cells during Baculovirus Infection Reveals Progressive Host Proteome Changes and Its Regulation by Viral miRNA. Sci. Rep. 2017, 7, 10902. [Google Scholar] [CrossRef] [PubMed]
- Cock, P.J.A.; Antao, T.; Chang, J.T.; Chapman, B.A.; Cox, C.J.; Dalke, A.; Friedberg, I.; Hamelryck, T.; Kauff, F.; Wilczynski, B.; et al. Biopython: Freely Available Python Tools for Computational Molecular Biology and Bioinformatics. Bioinformatics 2009, 25, 1422–1423. [Google Scholar] [CrossRef] [PubMed]
- Bellaousov, S.; Reuter, J.S.; Seetin, M.G.; Mathews, D.H. RNAstructure: Web Servers for RNA Secondary Structure Prediction and Analysis. Nucleic Acids Res. 2013, 41, W471–W474. [Google Scholar] [CrossRef] [PubMed]
- Will, S.; Joshi, T.; Hofacker, I.L.; Stadler, P.F.; Backofen, R. LocARNA-P: Accurate Boundary Prediction and Improved Detection of Structural RNAs. RNA 2012, 18, 900–914. [Google Scholar] [CrossRef]
- Medley, J.C.; Panzade, G.; Zinovyeva, A.Y. microRNA Strand Selection: Unwinding the Rules. WIREs RNA 2021, 12, e1627. [Google Scholar] [CrossRef]
- Reuter, J.S.; Mathews, D.H. RNAstructure: Software for RNA secondary structure prediction and analysis. BMC Bioinform. 2010, 11, 129. [Google Scholar] [CrossRef]
- Singh, C.P. Role of microRNAs in insect-baculovirus interactions. Insect Biochem. Mol. Biol. 2020, 127, 103459. [Google Scholar] [CrossRef]
- Zuker, M. Mfold Web Server for Nucleic Acid Folding and Hybridization Prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Enright, A.J.; John, B.; Gaul, U.; Tuschl, T.; Sander, C.; Marks, D.S. MicroRNA Targets in Drosophila. Genome Biol. 2003, 5, R1. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. miRDB: An Online Database for Prediction of Functional microRNA Targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef]
- Patil, A.H.; Halushka, M.K. miRge3.0: A Comprehensive microRNA and tRF Sequencing Analysis Pipeline. NAR Genom. Bioinform. 2021, 3, lqab068. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-Y.; Lin, Y.-C.-D.; Cui, S.; Huang, Y.; Tang, Y.; Xu, J.; Bao, J.; Li, Y.; Wen, J.; Zuo, H.; et al. miRTarBase Update 2022: An Informative Resource for Experimentally Validated miRNA–Target Interactions. Nucleic Acids Res. 2022, 50, D222–D230. [Google Scholar] [CrossRef] [PubMed]
- Hastie, T.; Tibshirani, R.; Narasimhan, B.; Chu, G. Impute: Imputation for Microarray Data. R Package Version 1.54.0. Available online: https://bioconductor.org/packages/release/bioc/html/impute.html (accessed on 27 November 2023).
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, M.; Iovino, N.; Unnerstall, U.; Gaul, U.; Segal, E. The Role of Site Accessibility in microRNA Target Recognition. Nat. Genet. 2007, 39, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Kalvari, I.; Nawrocki, E.P.; Ontiveros-Palacios, N.; Argasinska, J.; Lamkiewicz, K.; Marz, M.; Griffiths-Jones, S.; Toffano-Nioche, C.; Gautheret, D.; Weinberg, Z.; et al. Rfam 14: Expanded Coverage of Metagenomic, Viral and microRNA Families. Nucleic Acids Res. 2021, 49, D192–D200. [Google Scholar] [CrossRef] [PubMed]
- Miranda, K.C.; Huynh, T.; Tay, Y.; Ang, Y.-S.; Tam, W.-L.; Thomson, A.M.; Lim, B.; Rigoutsos, I. A Pattern-Based Method for the Identification of MicroRNA Binding Sites and Their Corresponding Heteroduplexes. Cell 2006, 126, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- RNAcentral Consortium; Sweeney, B.A.; Petrov, A.I.; Ribas, C.E.; Finn, R.D.; Bateman, A.; Szymanski, M.; Karlowski, W.M.; Seemann, S.E.; Gorodkin, J.; et al. RNAcentral 2021: Secondary Structure Integration, Improved Sequence Search and New Member Databases. Nucleic Acids Res. 2021, 49, D212–D220. [Google Scholar] [CrossRef]
- Lorenz, R.; Bernhart, S.H.; Höner Zu Siederdissen, C.; Tafer, H.; Flamm, C.; Stadler, P.F.; Hofacker, I.L. ViennaRNA Package 2.0. Algorithms Mol. Biol. 2011, 6, 26. [Google Scholar] [CrossRef]
- Rehmsmeier, M.; Steffen, P.; Höchsmann, M.; Giegerich, R. Fast and Effective Prediction of microRNA/Target Duplexes. RNA 2004, 10, 1507–1517. [Google Scholar] [CrossRef]
- Karagkouni, D.; Paraskevopoulou, M.D.; Chatzopoulos, S.; Vlachos, I.S.; Tastsoglou, S.; Kanellos, I.; Papadimitriou, D.; Kavakiotis, I.; Maniou, S.; Skoufos, G.; et al. DIANA-TarBase v8: A Decade-Long Collection of Experimentally Supported miRNA–Gene Interactions. Nucleic Acids Res. 2018, 46, D239–D245. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting Effective microRNA Target Sites in Mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef] [PubMed]





| Bac-miR | Guide Strand (miRNA) | Passenger Strand | ||
|---|---|---|---|---|
| Exterior Loop | First Stack | Exterior Loop | First Stack | |
| bmnpv-miR-1 | −0.3 | −1.1 | −0.3 | −2.5 |
| bmnpv-miR-2 | −2 | −1.7 | −0.2 | −2.7 |
| bmnpv-miR-3 | −1.4 | −2.8 | −0.3 | 0 |
| bmnpv-miR-4 | −2 | −3.7 | −0.3 | −1.1 |
| BmNPV-miR-415 | 0.5 | −2.5 | −1.5 | −2.5 |
| AcMNPV-miR-1 | −0.2 | −2.7 | −1.3 | −3.7 |
| AcMNPV-miR-2 | −0.1 | 0 | −0.1 | −1.5 |
| AcMNPV-miR-3 | −1.9 | −3.8 | −0.3 | −3.8 |
| AcMNPV-miR-4 | 0.5 | −1.1 | −0.3 | −2.4 |
| AcMNPV-miR-5 | −1.9 | −2.8 | −0.4 | −1.2 |
| agmnpv-miR-4 | −1.9 | −3.7 | −0.1 | 0.5 |
| SpltNPV-miR-11684-3p | 0.4 | −1.7 | −0.7 | −3.5 |
| SpltNPV-miR-11698-3p | −0.4 | −1.7 | −0.5 | −1.7 |
| Tool (Type) * | Details (Last Update) | URL | Reference |
|---|---|---|---|
| Mfold (Soft) | Secondary Structure Prediction (2003) | http://www.unafold.org/mfold/applications/rna-folding-form.php (accessed on 27 November 2023) | [59] |
| miRanda (Soft) | Target Prediction (2005) | http://cbio.mskcc.org/miRNA2003/miranda.html (accessed on 27 November 2023) | [60] |
| miRbase (DB) | General (2018) | https://www.mirbase.org/ (accessed on 27 November 2023) | [35] |
| miRDB (DB) | Target predictions and functional annotations (2019) | https://mirdb.org/ (accessed on 27 November 2023) | [61] |
| miRge3.0 (Soft) | Comprehensive analysis of small RNA sequencing data (2023) | https://github.com/mhalushka/miRge3.0 (accessed on 27 November 2023) | [62] |
| miRTarBase (DB) | Experimental microRNA-target Interactions (2021) | https://mirtarbase.cuhk.edu.cn/ (accessed on 27 November 2023) | [63] |
| miRTarget (Soft) | Target Prediction (2021) | https://rdrr.io/bioc/miRSM/man/miRTarget.html (accessed on 27 November 2023) | [64,65] |
| PITA (Soft) | Target Prediction (2008) | https://genie.weizmann.ac.il/pubs/mir07/mir07_prediction.html (accessed on 27 November 2023) | [66] |
| Rfam (DB) | Structural RNAs (2022) | https://rfam.org/ (accessed on 27 November 2023) | [67] |
| RNA22 (Soft) | Target Prediction (2016) | https://cm.jefferson.edu/rna22/Interactive/ (accessed on 27 November 2023) | [68] |
| RNAcentral (DB) | Non-coding RNAs (2023) | https://rnacentral.org/ (accessed on 27 November 2023) | [69] |
| RNAfold (Soft) | Secondary Structure Prediction (2021) | http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi (accessed on27 November 2023) | [70] |
| RNAhybrid (Soft) | Target-microRNA hybridization prediction (2013) | https://bibiserv.cebitec.uni-bielefeld.de/rnahybrid (accessed on 27 November 2023) | [71] |
| TarBase (DB) | General (2018) | https://dianalab.e-ce.uth.gr/html/diana/web/index.php?r=tarbasev8%2Findex (accessed on 27 November 2023) | [72] |
| TargetScan (Soft) | Target Prediction (2018) | https://www.targetscan.org/vert_72/ (accessed on 27 November 2023) | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motta, L.F.; Cerrudo, C.S.; Belaich, M.N. A Comprehensive Study of MicroRNA in Baculoviruses. Int. J. Mol. Sci. 2024, 25, 603. https://doi.org/10.3390/ijms25010603
Motta LF, Cerrudo CS, Belaich MN. A Comprehensive Study of MicroRNA in Baculoviruses. International Journal of Molecular Sciences. 2024; 25(1):603. https://doi.org/10.3390/ijms25010603
Chicago/Turabian StyleMotta, Lucas Federico, Carolina Susana Cerrudo, and Mariano Nicolás Belaich. 2024. "A Comprehensive Study of MicroRNA in Baculoviruses" International Journal of Molecular Sciences 25, no. 1: 603. https://doi.org/10.3390/ijms25010603
APA StyleMotta, L. F., Cerrudo, C. S., & Belaich, M. N. (2024). A Comprehensive Study of MicroRNA in Baculoviruses. International Journal of Molecular Sciences, 25(1), 603. https://doi.org/10.3390/ijms25010603