2.1. Interactions between the Materials of Hybrid Systems in the Solid State
A crucial aspect of developing hybrid systems composed of various materials lies in determining the interactions between them. Differential scanning calorimetry (DSC) serves as a valuable tool for analyzing these interactions. The samples examined were pure compounds of P407, Tw80, MβCD, and HPβCD, as well as their mixtures, including Tw80/MβCD at a 70:30 ratio, Tw80/HPβCD at a 70:30 ratio, P407/Tw80 at a 70:30 ratio, P407/MβCD at an 80:20 ratio, P407/HPβCD at an 80:20 ratio, and (P407/Tw80)/MβCD and (P407/Tw80)/HPβCD at an 80:20 ratio. The calorimetric heating profiles obtained by DSC analysis of pure compounds and composed systems in the solid state are displayed in
Figure 2, and the associated calorimetric parameters are outlined in
Table S1 (Supplementary Materials). It should be noted that other techniques like Fourier-transform infrared spectroscopy (FTIR) are also used to study the interactions between the materials of hybrid systems in the solid state. We used DSC analysis due to the highly reproducible phase transitions in a temperature range, which are of paramount importance for the performance of studies in the development of hybrid systems [
44,
45].
The wide endothermic peak at 60 °C for Tw80 (
Supplementary Materials, Table S1) might align with Tw80’s cloud point at 65 °C, where the surfactant’s solubility in aqueous media significantly decreases. As a result, surfactant molecules aggregate, leading to phase separation and a cloudy appearance in the solution, as described by Pérez-González et al. [
46] and Al-Sabagh et al. [
47].
Regarding the DSC thermogram of pure MβCD, it exhibited one broad endothermic peak at 180 °C and another one at 75 °C, with a low value of enthalpy (
Supplementary Materials, Table S1). In contrast, HPβCD’s DSC analysis showed an endothermic peak at 147 °C (
Supplementary Materials, Table S1), possibly due to water loss from the crystal and the absence of a defined fusion event due to the lack of a crystalline structure [
48]. When comparing the two β-CD derivatives, it was noted that MβCD presents a higher main transition temperature (T
m) than HPβCD. Moreover, the pure compound of HPβCD exhibits a higher enthalpy value (
Supplementary Materials, Table S1), indicating that the MβCD derivative possesses greater resistance to temperature increases and requires more energy to reach its melting point, potentially due to stronger intermolecular secondary interactions. CDs’ dehydration process is characterized by the endothermic peaks observed in the DSC thermograms [
49]. Different studies have reported a wide range of peaks for MβCD [
50,
51,
52,
53] and HPβCD [
54,
55,
56,
57,
58]. These variations could be attributed to differences in compound purity and sample preparation methods.
The DSC curves of P407, displayed in
Figure 2, revealed a distinct, endothermic, symmetric peak at 57 °C, which corresponds to the melting point of the compound. This confirms that under these conditions, the substance is stable and does not undergo decomposition. These results are consistent with those reported in other studies [
59,
60,
61,
62,
63]. In addition, a secondary exothermic peak was observed at 158 °C, which is in agreement with the findings of Pawar and Pande [
64].
Regarding the P407/Tw80 mixture (
Figure 2), it was observed that the main transition temperature was displaced at a lower temperature (53 °C) and there was a significant reduction of ΔH in comparison to the pure polymer (
Supplementary Materials, Table S1), revealing interactions between P407 and the surfactant. The changes in thermal behavior observed in this mixture are driven by the amphiphilic characteristics of the materials, which enable them to interact with each other through hydrophilic and hydrophobic interactions. Specifically, the hydrophilic chains of P407 (i.e., PEO chains) interact with the head group of Tw80, while the hydrophobic PPO chains of the polymer interact with the hydrophobic subunits of the surfactant. The formation of some hydrogen bond or van der Waals interactions may also contribute to these changes. In line with previous studies [
65], the decline in enthalpy values observed in a DSC curve, associated with a particular component following the addition of another material, is indicative of strong interactions between the two compounds.
Comparing the DSC results for the P407/MβCD and P407/HPβCD mixtures to those of pure P407, a slight shift of the main transition temperature to a lower one (53 °C) was noted. Moreover, the polymer’s exothermic peak was replaced with a much broader one at a higher temperature (188 °C) and one other peak at 162 °C with low enthalpy (9.85) appeared for the P407/MβCD and P407/HPβCD mixtures, respectively.
The DSC analysis of the P407/Tw80/MβCD mixture (as depicted in
Figure 2) showed that the addition of MβCD to the binary system significantly impacted the polymer’s thermal behavior, as evidenced by the appearance of two new peaks: one exothermic peak at 153 °C and an endothermic peak with a low enthalpy at 210 °C. On the other hand, P407/Tw80/HPβCD displayed a completely different peak pattern since it caused only a slight displacement of the main transition to a higher temperature (55 °C). The observed differences in the DSC thermograms of the mixtures may be attributed to the interactions developed between the materials and may be indicative of the formation of an inclusion complex in the ternary systems. The development of an inclusion complex between polymers and CDs has also been proven by DSC analysis in other studies. Zhang et al. [
66] reported the creation of an inclusion complex between the hydrophobic groups of the polymer upon their integration into the non-polar cavity of β-CD. Assembly formation was achieved through a combination of van der Waals forces and hydrophobic interactions during the inclusion complexation process.
The differences observed in mixtures with MβCD and HPβCD may be attributed to the dissimilar hydrophobic nature of the β-CD’s derivatives. The modifications at the β-CD substitution led to different interactions between the compounds and, subsequently, to different thermal behaviors. The DSC curves of the analyzed mixtures were characterized by low values of ΔΤ
1/2 (half width at half peak height of the transition) (
Supplementary Materials, Table S1), proving favorable cooperativity between the compounds.
Overall, the findings of the DSC analysis demonstrated the existence of a range of interactions between the compounds, and the presence of the non-ionic surfactant and CDs greatly changed the polymer’s thermal behavior, likely due to the creation of an inclusion complex.
The findings of our DSC studies are also confirmed by literature data provided by FTIR analyses investigating the spectra of either the pure substances or their combinations [
67,
68,
69,
70]. For example, FTIR analysis of pure P407 showed absorption bands at 3000–2850 cm
−1, -CH
2 stretch corresponding to the bonds present in PEO and PPO blocks, and stretching vibrations of C-C bonds at 1100 cm
−1 and at 1380 cm
−1 due to the symmetric angular deformation of CH
3 [
67]. Similar findings have been reported by Lin and Chang [
68], who confirmed the interactions improved when P407 was combined with HPβCD. More specifically, a noticeable reduction in the intensity of the bands was noted, suggesting interactions between HPβCD and the copolymer, primarily due to van der Waals interactions. This observation led to the inference that P407 engages with the hydrophobic cavity of HPβCD. Also, Bin Jardan et al. [
69] used FTIR analysis to identify the interactions developed between the materials of their ternary inclusion complex (comprising a pterostilbene, β-CD, and P407). Indeed, they proved that the combination of the materials diminished the peaks’ intensities and caused displacements of peaks compared to those of pure compounds. Finally, the higher affinity of β-CD to the PPO block of P407 was shown through the FTIR spectrum conducted by Di Donato et al. [
70]. This extracted conclusion is supported by the observation that the characteristic bands of P407 spectra appeared with weakened intensity after the addition of high concentrations of β-CD.
2.2. Physicochemical Characterization of Systems in Aqueous Solutions—Evaluation of Their Biological and Physical Stability
On the first day of colloidal dispersion preparation (
Section 3.2.2), measurements of particle size, the corresponding percentage of each population in each sample (weight of peak), polydispersity index (PDI), and zeta potential (z-potential) were carried out using dynamic and electrophoretic light scattering (DLS and ELS, respectively). The acquired data are presented in
Table 1.
DLS analysis of the colloidal dispersion of P407 revealed the presence of a heterogeneous particle population. Specifically, three distinct populations were observed, exhibiting different average hydrodynamic radius (R
h) values. The smallest one constitutes 6% of the total population, exhibiting an average R
h of 4 nm. The intermediate one, representing 38% of the total population, displays an average R
h of 39 nm and the largest one (55% of the total particles’ population) presents an average R
h of approximately 600 nm (
Table 1). This indicates the presence of relatively large particles within the dispersion. In the literature, variations in the size of P407 particles have been reported [
71]. The size of P407 particles can vary depending on various factors, including the concentration of the polymer, the method of preparation, and the source or manufacturing company. Upon the addition of the surfactant (Tw80), the particle size was reduced, resulting in a mean R
h of 29 nm. This reduction suggests that the surfactant had a dispersing effect on the larger particles, leading to the formation of smaller particles in the dispersion. However, despite the decrease in average particle size, our analysis uncovered significant heterogeneity among the particles, which is evident from the broad curves observed in
Figure S1 (Supplementary Materials). Similar findings regarding the dispersing impact of the surfactant (Tw80) were previously documented by Kontogiannis et al. [
72]. Their investigation focused on the alterations in the particle size of poloxamer 188 following the addition of Tw80. Their outcomes revealed a noteworthy reduction in the system’s particle size; however, there was also an increase in PDI value due to the presence of distinct populations of particles in the suspension.
The addition of CDs resulted in a reduction in particle size heterogenicity within the system, as demonstrated by the narrow curves observed in the size distribution diagram (
Supplementary Materials, Figure S1). More specifically, upon the further addition of MβCD, the primary population of particles (corresponding to 97% of the total population in both hybrid systems) presented an average R
h equal to 104 nm, while the incorporation of HPβCD led to an R
h of 114 nm. Therefore, no significant distinctions in particle size were observed in ternary systems with these different β-CD derivatives. Furthermore, it was noted that the dominant population of ternary systems presented significantly decreased R
h values compared to those of the colloidal dispersion of P407 (
Table 1), which is also supported by the findings of Li et al. [
73]. They demonstrated that the presence of HPβCD could decrease the particle size of prepared polymeric nanoparticles and lead to the formation of nanoparticles with a uniform particulate distribution.
The ELS measurements of the created compositions in an aqueous solution showed a slightly negative surface potential (
Table 1). Specifically, the surface charges of P407 and P407/Tw80/MβCD were measured to be −20.5 mV and −12.9 mV, respectively, which means that a slight electrostatic repulsion between the particles must exist. It is worth mentioning that the z-potential values of P407/Tw80 and P407/Tw80/HPβCD were found to be close to zero, indicating that the particle surfaces were mostly neutral in terms of charge. These particle size changes confirmed the results obtained by DSC experiments, which evidenced alterations in the DSC curves of the systems, proving the existence of interactions between the compounds utilized, which also modify the particle size of the coassembled systems in aqueous solutions.
The utilization of DLS has been widely employed to examine the characteristics and performance of nanostructures within serum media [
74]. A mixture of fetal bovine serum (FBS) and phosphate buffer solution (PBS) at a weight ratio of 10:90 was used as a dispersion medium, simulating the physicochemical conditions of human plasma (
Supplementary Materials, Table S2). The presence of proteins, such as albumin, in the biological medium influences the properties of the systems and this aspect is worth investigating [
74]. In both ternary systems, the R
h of the systems’ main particle population increased and new populations emerged. As illustrated in
Figure S2 (Supplementary Materials), the systems’ size distribution curves were broadened, indicating a high degree of polydispersity within the systems’ population, which is likely attributed to serum-induced aggregation. These results indicate the interactions between the system and plasma proteins improved. Based on the experimental findings mentioned above, it can be inferred that the hybrid nanostructures exhibit a certain degree of stealth characteristics and biological stability, attributed to the triblock P407 copolymer. The above results show that the P407 copolymer contains PEO chains, forming a hydrophilic corona which serves as a protective shield for the nanostructures against environmental factors. In a similar context, Chountoulesi et al. [
74] demonstrated the corona effect and steric stabilization of P407, which effectively protected their nanosystems from the presence of albumin in FBS.
Additionally, the formulations did not show any significant alterations in size when exposed to conditions that mimic the pH and temperature conditions of the human nasal cavity. Specifically, at a pH of 5.6 and a temperature of 34 °C, the systems retained their initial dimensions without observable modifications (
Supplementary Materials, Table S2). These findings strongly suggest that the formulations remain structurally intact and exhibit stability when exposed to simulated nasal cavity conditions, whereas the PEO chains partially impart biological stability to the composed structures.
The ternary systems’ stability was also evaluated by performing DLS measurements on dispersions that had been stored in glass vials at 4 °C in the refrigerator over a period of 30 days. This temperature is well-established for its ability to slow degradation processes and maintain colloidal integrity, making it a common choice in the determination of the kinetic stability of prepared systems [
71,
75,
76,
77]. As depicted in
Figure S3 (Supplementary Materials), the systems remained stable during this period as their R
h did not change significantly. As previously noted, the PEO chains of P407 extend beyond the system, providing steric stabilization due to the hydrophilic corona of PEO chains [
74]. Additionally, according to Akbar et al. [
78], the hydrophobic PPO chains of P407 align with the non-aqueous domains of the systems. Simultaneously, the hydrophilic component of P407 (PEO chains) extends outward into the aqueous environment. This extension of the PEO block from the particle surface effectively prevents particle aggregation and avoids sedimentation phenomena.
It was postulated that the presence of electrostatic interactions between the particles in the P407/Tw80/MβCD hybrid system may have been responsible for the physical stability of the system, in addition to the property of steric repulsion provided by the hydrophilic corona of PEO chains [
79]. Indeed, the results obtained from the DLS experiments confirm this hypothesis.
2.3. Study of the Internal Hydrophilic/Hydrophobic Environment of the Hybrid Systems
Fluorescence spectroscopy was employed to gain insight into the internal structure of the prepared systems, utilizing pyrene as a hydrophobic probe, which has the capability of being encapsulated within the hydrophobic domains of amphiphilic polymeric structures formed in aqueous solutions. The ratio of intensities between the first and the third peak in the pyrene emission spectrum, I
1/I
3, serves as a sensitive measure of the polarity of the environment surrounding the probe. As outlined in the literature [
80], I
1/I
3 ratio values between 1 to 1.3 indicate the presence of a hydrophobic microenvironment, while values in the range of 1.7 to 1.9 typically correspond to a polar environment.
P407 is one of the most widely used thermoresponsive polymers [
81]. As such, it was deemed appropriate to conduct fluorescence spectroscopy experiments under varying temperature conditions, including room temperature (25 °C), human body temperature (37 °C), and a temperature of an abnormal condition (50 °C) [
74,
82]. The results are presented in
Table 2.
The results indicated that the addition of the surfactant did not have a significant impact on the micropolarity of the systems at 37 °C and 50 °C. However, at 25 °C, the micropolarity of the systems decreased due to interactions between the amphiphilic compounds. Moreover, the addition of β-CD derivatives increased the hydrophilicity of the systems, as evidenced by the increase in I1/I3 values attributed to the hydrophilic outer surfaces of CDs. Specifically, the P407/Tw80/MβCD system exhibited an elevated I1/I3 value at 25 °C and 37 °C and an almost unaffected I1/I3 value at 50 °C. Lastly, the incorporation of HPβCD caused a rise in hydrophilicity, most prominently at 50 °C. The temperature shift from 25 °C to 37 °C resulted in an increase in I1/I3 value, indicating a heightened hydrophilicity of the systems. The results showed that the highest increase in I1/I3 value occurred in the P407/Tw80 system. Indeed, at a temperature of 25 °C, the I1/I3 value was 1.19, whereas at a temperature of 37 °C, it increased to 1.31. On the other hand, the increase in temperature from 37 °C to 50 °C led to a decrease in I1/I3 value, indicating a shift towards increased hydrophobicity, except for the P407/Tw80/HPβCD system, which remained constant. Fluorescence spectroscopy demonstrated that P407/Tw80/MβCD was particularly sensitive to this change in temperature, as the I1/I3 value reduced from 1.35 (37 °C) to 1.20 (50 °C). Presumably, some reorganization of the components takes place in hybrid coassembled aggregates at higher temperatures.
These findings are consistent with the data obtained from DSC experiments. The DSC results showed that all systems containing P407 exhibited a main transition peak around 50 °C, indicating a change in their thermal behavior and hydrophobicity/hydrophilicity ratio at this temperature. Therefore, the results obtained from the fluorescence spectroscopy technique confirm that P407 is a thermoresponsive polymer whose properties are influenced by temperature. In turn, this affects the internal environment of the hybrid systems.
2.4. Thermal Characterization of Colloidal Dispersions in Aqueous Solutions and Surface Tensiometric Analysis
Figure 3A and
Table 3 depict the microcalorimetry (mDSC) and high-resolution ultrasound spectroscopy (HR-US) thermograms and thermodynamic parameters of systems containing P407 with or without the presence of Tw80, MβCD, and HPβCD, respectively.
A clear endothermic transition was observed at 28 °C in the thermograms of P407, which is recognized as its main transition temperature. The addition of Tween 80 markedly affected the thermal behavior of the polymer, as evidenced by a broader peak with a higher enthalpy (ΔH
P407/Tw80/MβCD = 0.220 J/g) and centered at around 24 °C. These changes indicate the presence of interactions between the polymer and the surfactant, driven by the amphiphilic nature of the compounds, enabling the formation of both hydrophilic and hydrophobic interactions. Furthermore, the presence of MβCD and HPβCD in the P407/Tw80 system led to a slight displacement of the main transition temperature to lower values and the appearance of broader peaks, as seen from the remarkable reduction in enthalpy (ΔH
P407/Tw80/MβCD = 0.116 J/g, ΔH
P407/Tw80/MβCD = 0.101 J/g) in comparison to that of the P407 sample (ΔH
P407 = 0.175 J/g) (
Table 3). This observation could be attributed to the solubilization of the system due to the possible complexation of the compounds. It should be noted that the slight differences observed in the thermal behavior of the ternary systems with MβCD and HPβCD in the dispersion state may be due to the structures of CDs and their different water solubilities. The results of mDSC analysis revealed the presence of diverse interactions among the compounds of the system, which may be a result of self-assembly processes among P407, Tw80, and CDs.
To verify the main transitions observed through mDSC, a supplementary methodology known as HR-US was utilized (as represented in
Figure 3B). The analysis of the data obtained from HR-US, measuring the variation in sound speed over temperature, revealed that the transition temperatures for all systems ranged from 27 °C to 30 °C, highlighting again the effect of the presence of Tw80, MβCD, and HPβCD on the main thermal transition of P407.
The observations from the above techniques indicated that there are dissimilarities in the thermotropic characteristics of the prepared systems when compared to those obtained by DSC. These differences can be attributed to variations in the heating rates employed in the methods and the different states of the systems examined. In DSC, the interactions between the pure compounds were studied in the solid state. In contrast, the mDSC and HR-US techniques were utilized to analyze the particles in the dispersion state, where self-assembly or coassembly processes could potentially have taken place. Consequently, the presence of steric interactions resulting from the hydrophilic PEO chains led to the formation of colloidal particles exhibiting distinct thermal behavior in the dispersed liquid state.
Surface tension (γ) is a measure of the effectiveness of a surfactant in decreasing air–water surface tension [
83]. The calculated surface tension values for the prepared systems are presented in
Table 3. The value of γ for P407 was found to be 39 mN/m. The addition of Tween 80, as well as the further incorporation of HPβCD, did not have a significant impact on the value of γ for P407. With regards to the addition of MβCD, the surface tension values for the system remained largely constant and did not appear to be directly affected by the presence of MβCD. Overall, the results of the tensiometric analysis demonstrated that the presence of the surfactant and β-CD derivatives did not cause a significant alteration in γ values for P407-containing hybrid systems.
2.5. Morphological Characterization of Hybrid Systems
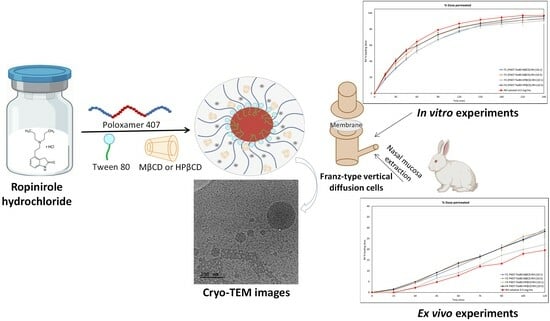
The morphological characteristics and dimensions of the prepared hybrid structures were also investigated using cryogenic transmission electron microscopy (cryo-TEM) images. The micrographs obtained from the analysis reveal the presence of spherical particles within the ternary systems (
Figure 4).
Specifically, the P407/Tw80/MβCD system exhibited particles with sizes ranging from 30 to 250 nm. The size of spherical particles in the P407/Tw80/HPβCD system was found to be 40–115 nm. Furthermore, it is imperative to take into consideration that the systems’ colloidal concentration (10 mg/mL) may influence the particles’ structural morphology. The observed shape asymmetry could also have arisen from the heterogeneous incorporation of triblock copolymer chains within the polymer/surfactant/CD inclusion complex. The results of the DSC, mDSC, and HR-US analyses demonstrated a change in the materials’ thermal behavior and the possible formation of an inclusion complex comprising the polymer, surfactant, and CD, with strong interactions observed between these components, likely due to the hydrophobic interior and hydrophilic exterior of the inclusion complex. This complex is illustrated as the spherical particle observed in cryo-TEM images.
The structures visualized in cryo-TEM images present differences in the average R
h values of the samples determined by intensity-weighted measurements in DLS. Besides the fact that both techniques are used to study particle size in structures, they are based on fundamentally different principles, which can lead to differences in the particle size measurements they provide. The cryo-TEM technique offers the advantage of investigating colloids directly in the vitrified, frozen-hydrated state following the plunge freezing of dispersions. This unique approach closely resembles the colloids’ native state, enabling the revelation of valuable insights into their internal and three-dimensional structure [
84]. In contrast, DLS measures the particle size of hybrid colloidal dispersions in the solution state in which self-assembly phenomena take place. It should be noted that there are some differences in the average size of nanoparticulate systems between DLS and cryo-TEM [
85,
86]. These differences are strongly associated with the solution state of the matter in DLS and the interactions of each particle with its neighboring particles (attractive or repulsive interactions). In other words, this discrepancy is also attributed to the swelling and Brownian motion of nanoparticles in the dispersion medium during DLS measurements, whereas TEM observations reflect the diameter of the solid state of the particles.
To conclude, cryo-TEM examines colloids in a frozen-hydrated state after plunge freezing, while DLS investigates the particle size of systems in a solution state. Particle sizes assessed by these two methods vary due to differences in their underlying principles [
85,
86].
2.6. Cytotoxicity of Colloidal Dispersions
The toxicity profiles of all the prepared systems were evaluated and they are presented in
Figure 5. Cell viability was expressed as a percentage (%) of cell viability ± standard deviation (SD) between two experiments. The results then underwent statistical analysis, as described below in
Section 3.2.15. The analysis of the data revealed that all systems displayed high cell viability at lower concentrations (25, 50, and 100 μg/mL), with values exceeding 90%. As the concentration of the systems increased, cell viability exhibited a proportional decrease, reaching 63–70% at the highest tested concentration of 500 µg/mL.
It is noteworthy that the inclusion of HPβCD had a positive impact on the toxicity profile of the prepared hybrid systems. Specifically, the ternary system containing HPβCD exhibited the highest cell viability at the highest concentrations tested (84% and 78% at concentrations of 400 and 500 µg/mL, respectively). The superior cell viability of P407/Tw80/HPβCD is statistically significant at concentrations of 300 and 500 µg/mL, compared to either P407 alone or in combination with Tw80 [
p < 0.05, 95% confidence interval (CI)], as well as to the ternary systems containing MβCD (
p < 0.05, 95% CI). In contrast, the presence of MβCD did not have any impact on cell viability compared to either P407 alone (
p > 0.05, 95% CI) or in combination with Tw80 (
p > 0.05, 95% CI), resulting in decreased cell viability, with the lowest observed value (63%) at a concentration of 500 µg/mL. It is known that nanotoxicity is influenced by multiple parameters, such as size and shape, which appear to be major contributors to cell internalization and cytotoxicity [
87].
Overall, the results of the MTT assay revealed that at low concentrations (25–200 μg/mL), all these combinations can be considered biocompatible, since the exhibited cell viability higher than 80%. Notably, the hybrid system containing HPβCD was also deemed biocompatible, even at higher concentrations (300 and 400 μg/mL). However, as the dose was increased, a correlation between dose and cytotoxicity was observed, with the degree of cytotoxicity varying based on the materials’ composition. As a result, it was found that all systems displayed dose-dependent and material-dependent toxicity on HEK293 cell lines. The findings from these toxicity studies will serve as a guide for evaluating the prepared systems as carriers for drug delivery.
2.7. Preformulation Studies and Physicochemical Characterization of RH-Containing Systems
RH served as the model drug for investigating the loading and release properties of the prepared hybrid polymer/surfactant/CD systems. The results taken from the DSC analysis for the P407/Tw80/MβCD/RH and P407/Tw80/HPβCD/RH formulations with varying concentrations of the API are presented in
Figure 6 and
Table S3 (Supplementary Materials).
Regarding the DSC results obtained for the pure RH compound, a sharp endothermic peak was observed at 249 °C, with a high value of enthalpy (ΔH = −22.69 KJ mol
−1). This peak is indicative of RH’s melting point and serves as evidence of its high crystallinity, which is consistent with other studies [
88,
89,
90]. As we mentioned above, the thermogram of the P407/Tw80/MβCD system displayed a main intense endothermic peak at 53 °C—which may be attributed to the crystalline regions of the PEO polymer and corresponds to the melting point of P407—as well as a secondary exothermic peak at 153 °C and a ternary endothermic peak at 210 °C with a low value of enthalpy (ΔH = −1.64 KJ mol
−1). The DSC scan of the P407/Tw80/HPβCD system revealed a main intense endothermic peak at 55 °C with ΔH value equal to −32.29 KJ mol
−1.
According to the results of DSC experiments for P407/Tw80/MβCD with RH, the main transition temperature of P407 remained consistently stable at 49–50 °C across all weight ratios. However, with an increase in the weight ratio of RH, a reduction in the enthalpy value of the endothermic peak corresponding to P407 was observed. This finding suggests that the presence of the API may induce alterations in the thermotropic behavior of the P407/Tw80/MβCD hybrid system. Thus, the reduction in the enthalpy value as the system-to-RH weight ratio increases proves the interactions developed between the system and RH. Lazaratos et al. [
65] reported similar observations. Notably, the diminished enthalpy value in the DSC curve of dipalmitoylphosphatidylcholine, as the amount of polyarabic acid increased, suggested a range of hydration forces and interactions between the polysaccharide chains of the nanoparticles and the polar head groups of the dipalmitoylphosphatidylcholine lipid bilayer.
RH’s endothermic peak changed as a function of increasing weight ratios of the drug. Moreover, as the system (P407/Tw80/CD) ratio in the formulation was increased, the sharpness of the drug’s endothermic peak was decreased. A displacement of RH’s transition temperature to lower values was also observed in all ratios, varying from 12 °C to 44 °C, as depicted in
Figure 6 and
Table S3 (Supplementary Materials). This alteration indicated a partial loss of crystallinity observed in RH upon its integration into the hybrid complex, a finding supported by Hussein et al. [
90]. A displacement of its transition temperature to lower values was observed in all ratios, varying from 12 °C to 44 °C. At the lowest concentration of the drug, the peak of RH was converted into a broadened exothermic peak at 221 °C, which was also proven by the extremely high ΔT
1/2 value (50.62 °C) (
Supplementary Materials, Table S3). This may indicate that the drug was uniformly dispersed at the molecular level within the hybrid system [
90] or that its concentration was too low to be detected by DSC. The highest shift of the drug’s peak was observed at a weight ratio of system/RH equal to 10:0.5. The corresponding thermogram presented an endothermic peak at 205 °C with a significantly reduced enthalpy value (ΔH = −1.42 KJ mol
−1). At weight ratios of system/RH of 10:1 and 10:5, the drug’s transition temperatures were decreased by 12–15 °C (T
m = 234 °C and 237 °C, respectively), whereas ΔH values were reduced to −1.48 KJ mol
−1 and −7.49 KJ mol
−1. The ratio equal to 10:1 showed excellent cooperativity of the materials, as inferred from the low value of ΔT
1/2 (2.28 °C). Upon DSC analysis of the system with the highest RH concentration, a notable transformation of the main endothermic peak was observed, resulting in the presence of two new endothermic peaks at 231 °C and 241 °C, exhibiting ΔH values of −9.12 KJ mol
−1 and −3.17 KJ mol
−1, respectively. These observations suggest that the system may have reached its maximum capacity for drug incorporation at a weight ratio of 10:1.
DSC analyses of P407/Tw80/HPβCD with RH revealed a minor downward shift in the primary transition temperature, with a decrease of 5–7 °C observed across all weight ratios. However, with an increase in the weight ratio of RH, a reduction in the enthalpy value of the main endothermic peak was observed. These findings suggest that the presence of the API may have induced alterations in the thermotropic behavior of the P407/Tw80/HPβCD hybrid system. Notably, at the lowest concentration of the drug (system-to-RH weight ratio equal to 10:0.1), a new endothermic peak, characterized by Tm = 170 °C and ΔH value equal to −4.24, emerged. Likewise, as with P407/Tw80/MβCD, this suggests that the drug was uniformly distributed at the molecular level within the hybrid system, or its concentration was too low to be detected efficiently by DSC. At a weight ratio of 10:0.5, no discernible curves associated with RH were identified, likely due to the extremely low drug concentration, which fell below the detectable limit of DSC. At system-to-RH weight ratios of 10:1, 10:5, and 10:10, the drug’s transition temperature decreased by 11 °C to 32 °C (Tm equal to 238 °C, 217 °C, and 226 °C, respectively), while the enthalpy value increased in line with the concentration of RH.
When comparing interactions in RH ternary systems utilizing different derivatives of β-CD, it is evident that, in both cases, an increase in the concentration of RH resulted in elevated ΔH values for the RH curve. Simultaneously, the corresponding enthalpy value of the P407 curve diminished. Additionally, at low concentrations of RH, the absence of any discernible curve corresponding to the drug may be attributed to the uniform molecular distribution of the drug within the hybrid system or a concentration too low to be efficiently detected by DSC. The cooperativity between materials is notably enhanced at weight ratios of system/RH of 10:1 and 10:5. Moreover, RH systems with MβCD exhibit superior cooperativity between compounds, as evidenced by lower ΔT1/2 values compared to the corresponding ones in systems with HPβCD.
Overall, based on the results of the DSC experiments, a multitude of interactions arose between the hybrid systems and the drug. In our opinion, the most promising weight ratios for further investigation are 10:1 and 10:5, as they demonstrated superior cooperativity between the materials, as evidenced by the lower values of ΔT1/2 observed in all peaks in comparison with the other weight ratios (especially for the P407/Tw80/MβCD/RH system), as well as the fact that at these weight ratios, the highest incorporation of the drug was achieved.
For this reason, hybrid ternary systems with RH at weight ratios of 10:1 and 10:5 were prepared by the thin-film hydration method (
Section 3.2.2). DLS measurements were conducted on the day of their preparation and the results are presented in
Table 4.
In the case of (P407/Tw80/MβCD)/RH at the weight ratio of 10:1, the results revealed a dominant population of 92% with an R
h of around 99 nm. Additionally, a minor population (7% of the total particle population) with an R
h of 4 nm was observed. Regarding the weight ratio of 10:5, DLS analysis showed a different particle distribution, revealing a population with an R
h of 211 nm (80% of the total particle population) and another with an R
h of 8 nm (20% of the total particle population). The addition of RH led to an increase in particle heterogeneity, as illustrated by the broad curves shown in
Figure S4 (Supplementary Materials) in both cases. These results suggest that the specific weight ratios of the components in the system influence particle size distribution, potentially due to different interactions and coassembly behaviors among the components. These outcomes align with the findings of Kuplennik and Sosnik [
29], who showed that variations in system-to-API weight ratio can either increase or decrease the particle size and PDI value of fabricated nanoparticles.
The P407/Tw80/HPβCD/RH systems comprised two populations. The smaller population, constituting 12% and 20% of the total, demonstrated an R
h of 6 nm in the systems with weight ratios of (P407/Tw80/HPβCD)/RH of 10:1 and 10:5
w/
w, respectively. The dominant population, representing 88% and 80% of the total in the corresponding systems, exhibited an R
h of 60 nm. The addition of RH to the P407/Tw80/HPβCD system resulted in a reduction in the particle size of both populations, accompanied by an augmentation in the system’s heterogeneity. This increase in heterogeneity was evident in the rise in the PDI value, as evidenced by the broadened curves illustrated in the DLS diagrams (
Supplementary Materials, Figure S4B). As a result, the two distinct β-CD derivatives facilitated a range of diverse interactions, leading to the formation of various bonds within the system and the RH. Consequently, this disparity ultimately contributed to differing effects on the particle size of the systems.
Figure 7 illustrates a potential configuration of the RH systems, based on the results obtained from all the applied techniques.
2.9. RH’s Release from the Hybrid Formulations by In Vitro Diffusion Experiments
In vitro release experiments of RH from the prepared formulations were performed using diffusion Franz cells and regenerated cellulose membranes with defined pore sizes as diffusion barrier. A molecular mass cut-off of 1000 Da enabled the permeation of free RH, blocking the excipients and/or the possible derived complexes in case of interactions occurring among the components of each formulation. The amount diffused is expressed as a percentage of the loading dose. RH is a freely soluble compound, and therefore, no differences were expected between the release profiles of the prepared formulations.
Figure 8 presents the time profiles of the cumulative amount of permeated RH per unit area and the amount of permeated RH as a percentage of the loading dose for the prepared formulations and the corresponding control solution with a concentration of 0.5 mg/mL.
Table 5 provides information on the percentage (%) of RH loading dose permeated for the tested formulations, the mass balance of RH in each formulation, and the percentage (%) of the RH loading dose retained by the cellulose membrane. All samples were analyzed using high-performance liquid chromatography (HPLC)-PDA (
Section 3.2.13).
No significant differences [p > 0.05, 95% CI among the same time points] were observed between formulations with the same weight ratios of the components and different β-CD derivatives (F1 vs. F3 and F2 vs. F4).
Regarding the percentage of the loading dose permeated over time, it exhibited significant variations among formulations with different concentrations of RH during the experiment. Formulations F2 and F4 (containing either MβCD or HPβCD, respectively, and an RH concentration of 5 mg/mL), demonstrated a more pronounced release profile of the drug compared to formulations F1 and F3, which contained either MβCD or HPβCD, respectively, and an RH concentration of 1 mg/mL. The most substantial differences (p < 0.05, 95% CI) were observed between formulations F3 and F4 from the beginning of the experiment up to 45 min.
When comparing the prepared formulations with the control solution (0.5 mg/mL, PBS pH = 5.6), it is noteworthy that the release profile of F4 and F2 closely resembles that of the RH solution. Notably, F4 displayed the highest cumulative amount and release profile among all colloidal dispersions throughout the entire duration of the experiment. Statistical analysis revealed no significant differences (p > 0.05, 95% CI) for formulations F4 and F2 compared to the control RH solution (0.5 mg/mL, PBS pH = 5.6). However, formulations F1 and F3 (containing MβCD and HPβCD, respectively, and an RH concentration of 1 mg/mL) exhibited statistically significantly lower release profiles than the control solution (0.5 mg/mL, PBS pH = 5.6) (p < 0.05, 95% CI).
By utilizing Equation (1), the flux (J) values for the manufactured formulations were calculated and ranged from 4.9 × 10
−4 ± 5.1 × 10
−5 to 5.9 × 10
−4 ± 8.9 × 10
−5 μg/cm
2/min (
Supplementary Materials, Table S4). Additionally, the R-square values ranged from 0.8978 ± 0.0092 to 0.9572 ± 0.0054, revealing a strong correlation between the concentration of the substance and the rate of release, which is consistent with the characteristics of a first-order reaction (
Supplementary Materials, Table S4). Furthermore, the quantification of retained RH content within the cellulose membrane fell within the range of 0.37 ± 0.02 to 0.50 ± 0.07 of the loading doses.
Overall, the prepared hybrid RH systems exhibit a remarkably high mass balance of up to 90% in all cases (
Figure 8,
Table 5). The most rapid increase in release rate was achieved at a concentration of RH equal to 5 mg/mL, indicating that different weight ratios of the components influence the rate and extent of drug release. Notably, the most beneficial release profile, observed in formulations F2 and F4 (system/RH weight ratio equal to 10:5), can be attributed to the improved interactions among the components of each formulation, effectively facilitating the diffusion of the drug into the membrane.
To conclude, as RH is classified as a BCS class III drug, presenting high water solubility, findings from the in vitro experiments did not permit the identification of crucial differences among the release profiles of the formulations, demonstrating a remarkably high mass balance of up to 90% in all cases. Therefore, it can be concluded that the presence of particles did not significantly affect the solubility of RH compared to the bulk form of the drug. Additional investigation involving ex vivo permeation experiments is needed to corroborate these findings.
2.10. RH’s Ex Vivo Mucosal Permeation from the Hybrid Formulations
The results of ex vivo permeation experiments are presented in
Figure 9. The quantification of RH remaining in the nasal mucosa barrier revealed that 17.44 ± 2.12% of the loading dose was retained on average by the biological membrane. The membrane retention data of each formulation expressed as the percentage (%) of the loading dose retained by the nasal mucosa barrier ± standard error of the mean (SEM) are included in
Table 6.
The RH solution (0.5 mg/mL, PBS pH = 5.6) presented a percentage of the loading dose permeated through the nasal mucosa equal to 19.62 ± 0.53. Formulations F1, F2, and F4 enhanced the permeability of RH up to 50% compared to the RH solution. In contrast, formulation F3 displayed a less favorable permeation profile, closely resembling that of the RH solution. Formulations F2 and F4, with a hybrid-system-to-RH weight ratio of 10:5, demonstrated a notably enhanced profile, as depicted in
Figure 9. No significant differences (
p > 0.05, 95% CI) were observed between them, suggesting that the two CD derivatives did not exert a differential effect on permeation through the nasal mucosa.
The J across the nasal mucosa barrier for all the tested formulations varied from 1.7 × 10
−4 ± 1.0 × 10
−5 to 2.0 × 10
−4 ± 1.0 × 10
−5 (μg/cm
2/min), while the apparent permeability (
Papp) ranged from 0.35 to 0.40 (cm/min). The values of J and
Papp for each formulation are included in
Table S4 (Supplementary Materials).
It has been reported that P407 can enhance drug transport through the nasal mucosa by decreasing the viscosity and elasticity of the mucus [
91,
92,
93]. Saha et al. [
93] developed a nanosuspension stabilized with P407, incorporating rotigotine for intranasal administration. The study of the in vitro dissolution of the optimized formulation demonstrated a rapid dissolution rate, with 95% of the cumulative drug dissolving within the first 15 min. The rotigotine nanosuspension exhibited a 20-fold increase in nasal permeation compared to a pure drug suspension, using goat nasal mucosa as a diffusion barrier. In addition, the intranasal administration in mice of an optimized zotepine formulation created by Pailla et al. [
92], comprising P407 (0.3%
w/v), hydroxypropyl methyl cellulose E15 (0.3%
w/v), and soya lecithin (0.4%
w/v), exhibited significantly elevated brain concentrations, with an 8.6-fold increase and a 10.79-fold rise in AUC
0–24h compared to intravenous zotepine solution.
Lin et al. [
13] previously reported a synergistic effect between P407 and CDs. Their study focused on the intranasal delivery of an antiparkinsonian drug using a thermosensitive gel formulation containing P407 (20%), poloxamer 188 (1%), PEG-6000 (1%), and HPβCD (3%). Pharmacokinetic analysis revealed a noteworthy 1.6-fold increase in drug bioavailability and a 2.1-fold increase in brain targeting compared to oral administration. This highlights the potential of combining polymers, particularly P407 in the highest proportion, with CDs to enhance drug bioavailability following intranasal administration.
Therefore, the materials of our system, namely P407, Tw80, and CDs, serve as permeation enhancers by interacting with the mucosal epithelium, thereby influencing the integrity of the barrier and ultimately enhancing RH permeability. This suggests a positive impact resulting from the synergistic interactions of the three components.
2.11. Comparison of Polymer/Surfactant/Cyclodextrin System with other Hybrid Formulations for Nose-to-Brain Delivery of RH
The effectiveness of nose-to-brain RH delivery has been given increasing attention recently. Several types of hybrid nanoparticles have already been developed for the nasal administration of RH [
94,
95,
96,
97]. In all cases, the added value of these systems comes from the combination of materials of a different nature, i.e., polymers, CDs, or lipids, which enhances the permeability of RH, leading to site-specific targeting as well as controlled release. From a technological point of view, different types of nanoparticles, ranging from mucoadhesive ones to nanocomplexes, can lead to superior biological behavior, too [
94,
95,
96,
97].
More specifically, Chatzitaki et al. [
94] fabricated mucoadhesive nanoparticles by combining poly (lactic-co-glycolic acid) and chitosan. These nanoparticles were used for the encapsulation of RH and demonstrated the ability to enhance the apparent ex vivo permeability of RH across sheep nasal mucosa by 3.22 times.
An improvement in RH nasal membrane permeability through sheep nasal mucosa was also reported by Pardeshi and Belgamwar [
95], who fabricated a nanoplex by using dextran sulfate sodium. RH exhibited a controlled release pattern from the nanoplex, reaching a cumulative release of 44.98% ± 1.7% over the 24 h experimental duration.
Additionally, Jafarieh et al. [
96] formulated a polymeric nanoparticulate carrier consisting of chitosan nanoparticles containing RH to facilitate targeted delivery to the brain. The in vitro release pattern of RH from the formulation demonstrated an initial rapid release of around 35% within 1 h, followed by a gradual and sustained drug release over 18 h, achieving a cumulative release of 89.18 ± 2.43%. The ex vivo experiments, using porcine nasal mucosa as a permeation barrier, revealed at least a twofold increase in the amount of permeated RH for the RH NPs compared to the control RH solution.
To the same context, Pardeshi et al. [
97] also designed and fabricated polymer–lipid hybrid nanoparticles. Their in vitro release study showed a sustained release profile of RH from the tested formulation, whereas the percentage of the drug permeated across sheep nasal mucosa after 8 h was found to be 69.88 ± 1.48%. In vivo pharmacodynamic studies confirmed that the tested nasal formulation exhibited comparable therapeutic activity to the oral formulation, demonstrating effectiveness at a lower dosage. This suggests the potential to reduce both dose and dosing frequency and maximize the therapeutic index of RH.
Considering the above hybrid systems that were recently developed in the literature, it also should be noted that the excipients that are used in the present study are commercially available, which can lead to easier scale-up of the final formulations and reproducible physicochemical characterization [
98]. We did not select natural polymers, i.e., chitosan or dextran. There are several challenges in the use of these natural polymers in terms of their stability, scalability, and immunogenicity [
99]. However, direct comparisons with the present study’s results are not feasible due to variations in experimental conditions. Specifically, all previous studies used sheep and porcine nasal mucosa for ex vivo permeation experiments instead of rabbit nasal mucosa. Furthermore, the conduction of in vitro experiments differed in terms of RH loading doses, duration, and techniques used (method of dialysis bag).
Further studies are scheduled to evaluate the effect of dose and formulation on the in vitro release and ex vivo permeation of RH through rabbit nasal mucosa, as well as the in vivo serum and brain pharmacokinetic profiles of the developed formulations after nasal administration.