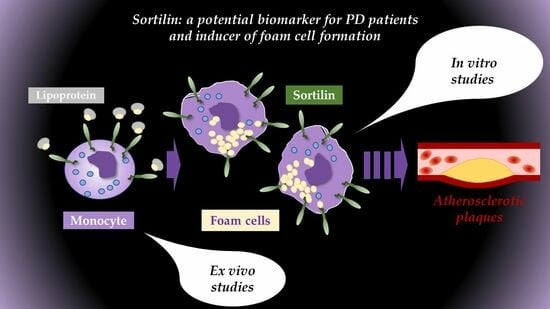
Sortilin Expression Levels and Peripheral Immunity: A Potential Biomarker for Segregation between Parkinson’s Disease Patients and Healthy Controls
Abstract
:1. Introduction
2. Results
2.1. Patient Demographics of the Main Cohort Study
2.2. Altered Immune Cell Populations in Peripheral Blood of PD Patients
2.3. Differential Sortilin Expression Levels on Monocytes from PD Patients and Correlation with Disease
2.4. In Vitro Functional Studies Differentiate PBMC-Derived Macrophages between Healthy Donors and PD Patients
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Blood Samples and Flow Cytometry Analysis
4.3. Human Peripheral Blood Mononuclear Cell-Derived Macrophages—Isolation and In Vitro Differentiation
4.4. Foam Cell Formation
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 509. [Google Scholar] [CrossRef]
- Moustafa, A.A.; Chakravarthy, S.; Phillips, J.R.; Gupta, A.; Keri, S.; Polner, B.; Frank, M.J.; Jahanshahi, M. Motor symptoms in Parkinson’s disease: A unified framework. Neurosci. Biobehav. Rev. 2016, 68, 727–740. [Google Scholar] [CrossRef]
- Bobela, W.; Aebischer, P.; Schneider, B.L. Alphalpha-Synuclein as a Mediator in the Interplay between Aging and Parkinson’s Disease. Biomolecules 2015, 5, 2675–2700. [Google Scholar] [CrossRef]
- Tsalenchuk, M.; Gentleman, S.M.; Marzi, S.J. Linking environmental risk factors with epigenetic mechanisms in Parkinson’s disease. NPJ Park. Dis. 2023, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Gu, X.; Wang, X. Alpha-Synuclein in Parkinson’s disease and advances in detection. Clin. Chim. Acta 2022, 529, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Dardiotis, E.; Xiromerisiou, G.; Hadjichristodoulou, C.; Tsatsakis, A.M.; Wilks, M.F.; Hadjigeorgiou, G.M. The interplay between environmental and genetic factors in Parkinson’s disease susceptibility: The evidence for pesticides. Toxicology 2013, 307, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Brockmann, K. Blood and Cerebrospinal Fluid Biomarkers of Inflammation in Parkinson’s Disease. J. Park. Dis. 2022, 12, S183–S200. [Google Scholar] [CrossRef] [PubMed]
- Xiromerisiou, G.; Marogianni, C.; Lampropoulos, I.C.; Dardiotis, E.; Speletas, M.; Ntavaroukas, P.; Androutsopoulou, A.; Kalala, F.; Grigoriadis, N.; Papoutsopoulou, S. Peripheral Inflammatory Markers TNF-alpha and CCL2 Revisited: Association with Parkinson’s Disease Severity. Int. J. Mol. Sci. 2022, 24, 264. [Google Scholar] [CrossRef] [PubMed]
- Scalzo, P.; Kümmer, A.; Cardoso, F.; Teixeira, A. Serum levels of interleukin-6 are elevated in patients with Parkinson’s disease and correlate with physical performance. Neurosci. Lett. 2010, 468, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, K.W.; Schuh, A.F.S.; Saute, J.; Townsend, R.; Fricke, D.; Leke, R.; Souza, D.O.; Portela, L.V.; Chaves, M.L.F.; Rieder, C.R.M. Interleukin-6 serum levels in patients with Parkinson’s disease. Neurochem. Res. 2009, 34, 1401–1404. [Google Scholar] [CrossRef]
- Dursun, E.; Gezen-Ak, D.; Hanağası, H.; Bilgiç, B.; Lohmann, E.; Ertan, S.; Atasoy, İ.L.; Alaylıoğlu, M.; Araz, Ö.S.; Önal, B.; et al. The interleukin 1 alpha, interleukin 1 beta, interleukin 6 and alpha-2-macroglobulin serum levels in patients with early or late onset Alzheimer’s disease, mild cognitive impairment or Parkinson’s disease. J. Neuroimmunol. 2015, 283, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Marogianni, C.; Sokratous, M.; Dardiotis, E.; Hadjigeorgiou, G.M.; Bogdanos, D.; Xiromerisiou, G. Neurodegeneration and Inflammation-An Interesting Interplay in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8421. [Google Scholar] [CrossRef] [PubMed]
- Becher, B.; Spath, S.; Goverman, J. Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 2017, 17, 49–59. [Google Scholar] [CrossRef]
- Tran, J.; Anastacio, H.; Bardy, C. Genetic predispositions of Parkinson’s disease revealed in patient-derived brain cells. NPJ Park. Dis. 2020, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Guadagnolo, D.; Piane, M.; Torrisi, M.R.; Pizzuti, A.; Petrucci, S. Genotype-Phenotype Correlations in Monogenic Parkinson Disease: A Review on Clinical and Molecular Findings. Front. Neurol. 2021, 12, 648588. [Google Scholar] [CrossRef] [PubMed]
- Nalls, M.A.; Pankratz, N.; Lill, C.M.; Do, C.B.; Hernandez, D.G.; Saad, M.; DeStefano, A.L.; Kara, E.; Bras, J.; Sharma, M.; et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet. 2014, 46, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.Q.; Zheng, R.; Liu, Y.; Ruan, Y.; Lin, Z.H.; Xue, N.J.; Chen, Y.; Zhang, B.R.; Pu, J.L. Parkin regulates microglial NLRP3 and represses neurodegeneration in Parkinson’s disease. Aging Cell 2023, 22, e13834. [Google Scholar] [CrossRef]
- Orr, C.F.; Rowe, D.B.; Mizuno, Y.; Mori, H.; Halliday, G.M. A possible role for humoral immunity in the pathogenesis of Parkinson’s disease. Brain 2005, 128 Pt 11, 2665–2674. [Google Scholar] [CrossRef]
- Calabresi, P.; Mechelli, A.; Natale, G.; Volpicelli-Daley, L.; Di Lazzaro, G.; Ghiglieri, V. Alpha-synuclein in Parkinson’s disease and other synucleinopathies: From overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 2023, 14, 176. [Google Scholar] [CrossRef]
- Xiromerisiou, G.; Bourinaris, T.; Houlden, H.; Lewis, P.A.; Senkevich, K.; Hammer, M.; Federoff, M.; Khan, A.; Spanaki, C.; Hadjigeorgiou, G.M.; et al. SORL1 mutation in a Greek family with Parkinson’s disease and dementia. Ann. Clin. Transl. Neurol. 2021, 8, 1961–1969. [Google Scholar] [CrossRef]
- Hermey, G. The Vps10p-domain receptor family. Cell Mol. Life Sci. 2009, 66, 2677–2689. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.M.; Naves, T.; Saada, S.; Pinet, S.; Vincent, F.; Lalloue, F.; Jauberteau, M.-O. The implications of sortilin/vps10p domain receptors in neurological and human diseases. CNS Neurol. Disord. Drug Targets 2014, 13, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.H.; Hansson, O.; Minthon, L.; Andreasen, N.; Blennow, K.; Zetterberg, H.; Skoog, I.; Wallin, A.; Nilsson, S.; Kettunen, P. A Genetic Variant of the Sortilin 1 Gene is Associated with Reduced Risk of Alzheimer’s Disease. J. Alzheimers Dis. 2016, 53, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Deng, Y.-P.; Yi, X.; Cao, H.-Y.; Zou, H.-Q.; Wang, X.; Liang, C.-R.; Wang, Y.-R.; Zhang, L.-L.; Gao, C.-Y.; et al. No association of SORT1 gene polymorphism with sporadic Alzheimer’s disease in the Chinese Han population. NeuroReport 2013, 24, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z. An Integrated Network Analysis of mRNA and Gene Expression Profiles in Parkinson’s Disease. Med. Sci. Monit. 2020, 26, e920846. [Google Scholar] [PubMed]
- Marsili, L.; Duque, K.R.; Bode, R.L.; Kauffman, M.A.; Espay, A.J. Uncovering Essential Tremor Genetics: The Promise of Long-Read Sequencing. Front. Neurol. 2022, 13, 821189. [Google Scholar] [CrossRef] [PubMed]
- Talbot, H.; Saada, S.; Naves, T.; Gallet, P.-F.; Fauchais, A.-L.; Jauberteau, M.-O. Regulatory Roles of Sortilin and SorLA in Immune-Related Processes. Front. Pharmacol. 2018, 9, 1507. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.; O’gaora, P.; James, W.G.; McClelland, S.; Fitzgerald, D.J.; Belton, O.; de Gaetano, M. SorLA modulates atheroprotective properties of CLA by regulating monocyte migration. Atherosclerosis 2010, 213, 400–407. [Google Scholar] [CrossRef]
- Herda, S.; Raczkowski, F.; Mittrücker, H.W.; Willimsky, G.; Gerlach, K.; Kühl, A.A.; Breiderhoff, T.; Willnow, T.E.; Dörken, B.; Höpken, U.E.; et al. The sorting receptor Sortilin exhibits a dual function in exocytic trafficking of interferon-gamma and granzyme A in T cells. Immunity 2012, 37, 854–866. [Google Scholar] [CrossRef]
- Elgueta, R.; Benson, M.J.; De Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef] [PubMed]
- Tentillier, N.; Etzerodt, A.; Olesen, M.N.; Rizalar, F.S.; Jacobsen, J.; Bender, D.; Moestrup, S.K.; Romero-Ramos, M. Anti-Inflammatory Modulation of Microglia via CD163-Targeted Glucocorticoids Protects Dopaminergic Neurons in the 6-OHDA Parkinson’s Disease Model. J. Neurosci. 2016, 36, 9375–9390. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Matsui, A.; Ohyagi, M.; Kikutake, C.; Harada, Y.; Iizuka-Koga, M.; Suyama, M.; Yoshimura, A.; Ito, M. In Vitro Generation of Brain Regulatory T Cells by Co-culturing With Astrocytes. Front. Immunol. 2022, 13, 960036. [Google Scholar] [CrossRef]
- Lauritsen, J.; Romero-Ramos, M. The systemic immune response in Parkinson’s disease: Focus on the peripheral immune component. Trends Neurosci. 2023, 46, 863–878. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yang, W.; Wei, H.; Dean, M.N.; Standaert, D.G.; Cutter, G.R.; Benveniste, E.N.; Qin, H. Dysregulation of the Adaptive Immune System in Patients with Early-Stage Parkinson Disease. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Gao, H.; Luo, Q.; Wang, P.; Yang, X. The correlation of lymphocyte subsets, natural killer cell, and Parkinson’s disease: A meta-analysis. Neurol. Sci. 2017, 38, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Meredith, E.J.; Holder, M.J.; Rosén, A.; Lee, A.D.; Dyer, M.J.S.; Barnes, N.M.; Gordon, J. Dopamine targets cycling B cells independent of receptors/transporter for oxidative attack: Implications for non-Hodgkin’s lymphoma. Proc. Natl. Acad. Sci. USA 2006, 103, 13485–13490. [Google Scholar] [CrossRef]
- Stevens, C.H.; Rowe, D.; Morel-Kopp, M.C.; Orr, C.; Russell, T.; Ranola, M.; Ward, C.; Halliday, G.M. Reduced T helper and B lymphocytes in Parkinson’s disease. J. Neuroimmunol. 2012, 252, 95–99. [Google Scholar] [CrossRef]
- Grozdanov, V.; Bliederhaeuser, C.; Ruf, W.P.; Roth, V.; Fundel-Clemens, K.; Zondler, L.; Brenner, D.; Martin-Villalba, A.; Hengerer, B.; Kassubek, J.; et al. Inflammatory dysregulation of blood monocytes in Parkinson’s disease patients. Acta Neuropathol. 2014, 128, 651–663. [Google Scholar] [CrossRef]
- Mussbacher, M.; Derler, M.; Basílio, J.; Schmid, J.A. NF-kappaB in monocytes and macrophages—An inflammatory master regulator in multitalented immune cells. Front. Immunol. 2023, 14, 1134661. [Google Scholar] [CrossRef]
- Chen, L.W.; Yung, K.K.L.; Chan, Y.S.; Shum, D.K.Y.; Bolam, J.P. The proNGF-p75NTR-sortilin signalling complex as new target for the therapeutic treatment of Parkinson’s disease. CNS Neurol. Disord. Drug Targets 2008, 7, 512–523. [Google Scholar] [CrossRef]
- Pallesen, L.T.; Vaegter, C.B. Sortilin and SorLA regulate neuronal sorting of trophic and dementia-linked proteins. Mol. Neurobiol. 2012, 45, 379–387. [Google Scholar] [CrossRef]
- Longo, F.M.; Yang, T.; Knowles, J.K.; Xie, Y.; Moore, L.A.; Massa, S.M. Small molecule neurotrophin receptor ligands: Novel strategies for targeting Alzheimer’s disease mechanisms. Curr. Alzheimer Res. 2007, 4, 503–506. [Google Scholar] [CrossRef]
- Clee, S.M.; Yandell, B.S.; Schueler, K.M.; Rabaglia, M.E.; Richards, O.C.; Raines, S.M.; Kabara, E.A.; Klass, D.M.; Mui, E.T.-K.; Stapleton, D.S.; et al. Positional cloning of Sorcs1, a type 2 diabetes quantitative trait locus. Nat. Genet. 2006, 38, 688–693. [Google Scholar] [CrossRef]
- Holdt, L.M.; Teupser, D. From genotype to phenotype in human atherosclerosis—Recent findings. Curr. Opin. Lipidol. 2013, 24, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Jia, B.; Xie, W.; Yang, J.; Lv, Y. Mechanism underlying the regulation of sortilin expression and its trafficking function. J. Cell Physiol. 2020, 235, 8958–8971. [Google Scholar] [CrossRef] [PubMed]
- Mitok, K.A.; Keller, M.P.; Attie, A.D. Sorting through the extensive and confusing roles of sortilin in metabolic disease. J. Lipid Res. 2022, 63, 100243. [Google Scholar] [CrossRef]
- Mortensen, M.B.; Kjolby, M.; Gunnersen, S.; Larsen, J.V.; Palmfeldt, J.; Falk, E.; Nykjaer, A.; Bentzon, J.F. Targeting sortilin in immune cells reduces proinflammatory cytokines and atherosclerosis. J. Clin. Investig. 2014, 124, 5317–5322. [Google Scholar] [CrossRef]
- Yabe-Wada, T.; Matsuba, S.; Takeda, K.; Sato, T.; Suyama, M.; Ohkawa, Y.; Takai, T.; Shi, H.; Philpott, C.C.; Nakamura, A. TLR signals posttranscriptionally regulate the cytokine trafficking mediator sortilin. Sci. Rep. 2016, 6, 26566. [Google Scholar] [CrossRef]
- Carlo, A.S. Sortilin, a novel APOE receptor implicated in Alzheimer disease. Prion 2013, 7, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lin, W.; Tian, L.; Di, B.; Yu, J.; Niu, X.; Liu, J. Oxidized low-density lipoprotein activates extracellular signal-regulated kinase signaling to downregulate sortilin expression in liver sinusoidal endothelial cells. J. Gastroenterol. Hepatol. 2021, 36, 2610–2618. [Google Scholar] [CrossRef] [PubMed]
- Xiang, P.; Blanchard, V.; Francis, G.A. Smooth Muscle Cell-Macrophage Interactions Leading to Foam Cell Formation in Atherosclerosis: Location, Location, Location. Front. Physiol. 2022, 13, 921597. [Google Scholar] [CrossRef] [PubMed]
- Lehr, H.A.; Krombach, F.; Münzing, S.; Bodlaj, R.; Glaubitt, S.I.; Seiffge, D.; Hübner, C.; Von Andrian, U.H.; Messmer, K. In vitro effects of oxidized low density lipoprotein on CD11b/CD18 and L-selectin presentation on neutrophils and monocytes with relevance for the in vivo situation. Am. J. Pathol. 1995, 146, 218–227. [Google Scholar] [PubMed]
- Patel, K.M.; Strong, A.; Tohyama, J.; Jin, X.; Morales, C.R.; Billheimer, J.; Millar, J.; Kruth, H.; Rader, D.J. Macrophage sortilin promotes LDL uptake, foam cell formation, and atherosclerosis. Circ. Res. 2015, 116, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and Its Impact on Cardiovascular Health: Focus on Atherosclerosis. Front. Pharmacol. 2020, 11, 613780. [Google Scholar] [CrossRef]
- Papoutsopoulou, S.; Burkitt, M.D.; Bergey, F.; England, H.; Hough, R.; Schmidt, L.; Spiller, D.G.; White, M.H.; Paszek, P.; Jackson, D.A.; et al. Macrophage-Specific NF-κB Activation Dynamics Can Segregate Inflammatory Bowel Disease Patients. Front. Immunol. 2019, 10, 2168. [Google Scholar] [CrossRef]
- Xu, S.; Huang, Y.; Xie, Y.; Lan, T.; Le, K.; Chen, J.; Chen, S.; Gao, S.; Xu, X.; Shen, X.; et al. Evaluation of foam cell formation in cultured macrophages: An improved method with Oil Red O staining and DiI-oxLDL uptake. Cytotechnology 2010, 62, 473–481. [Google Scholar] [CrossRef]






| Parkinson’s Disease (PD) Patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Sex | PD Type | Age | Age at Onset | Duration of Disease | BMI | UPDRS/Motor Scale | Drug Treatment | Comorbidities |
| 1 | F | SNCA (A53T) | 55 | 40 | 15 | 16.9 | 29 | Levodopa, Memantine Amantantin, antidepressants | PD dementia |
| 2 | M | Sporadic | 42 | 31 | 11 | 23.5 | 25 | Levodopa therapy, deep brain stimulation | None |
| 3 | M | Sporadic | 60 | 59 | 1 | 25.3 | 8 | Levodopa, Rasagelin | None |
| 4 | F | Sporadic | 68 | 66 | 2 | 29 | 8 | Dopamine agonist, Rasagelin | None |
| 5 | M | SORT1 (G379W) | 69 | 66 | 3 | 28.4 | 8 | Dopamine agonist, Safinamide, Levodopa, ACE inhibitors | Hypertension |
| 6 | F | Sporadic | 72 | 64 | 8 | 20.8 | 30 | Levodopa, Memantine | PD dementia |
| 7 | M | Sporadic | 68 | 64 | 4 | 26.6 | 9 | Dopamine agonist, Rasagelin | Atrial fibrillation |
| 8 | F | SNCA (A30P) | 67 | 62 | 5 | 28.1 | 18 | Levodopa, angiotensin II receptor blockers | Thyroidectomy Hypertension |
| 9 | F | SORT1 (G379W) | 77 | 75 | 3 | 26 | 12 | Dopamine agonist, Levodopa, Safinamide | None |
| 10 | M | SORT1 (G379W) | 60 | 57 | 3 | 30.4 | 12 | Dopamine agonist, Levodopa | Stroke |
| Healthy donors | |||||||||
| No | Sex | Age | BMI | Drug treatment | Noted minor conditions | ||||
| 1 | M | 22 | 22.7 | no | no | ||||
| 2 | M | 75 | 22.1 | ACE inhibitors | Hypertension | ||||
| 3 | F | 60 | 26.6 | no | no | ||||
| 4 | M | 36 | 27.8 | no | no | ||||
| 5 | M | 52 | 21.9 | Atorvastatin, Rosurvastatin | Hyperlipidemia | ||||
| 6 | F | 38 | 21.5 | no | no | ||||
| 7 | M | 68 | 27 | no | no | ||||
| 8 | F | 60 | 23.4 | no | no | ||||
| 9 | F | 67 | 25.4 | no | no | ||||
| 10 | M | 74 | 25.8 | no | no | ||||
| 11 | M | 75 | 24.8 | no | no | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgoula, M.; Ntavaroukas, P.; Androutsopoulou, A.; Xiromerisiou, G.; Kalala, F.; Speletas, M.; Asprodini, E.; Vasilaki, A.; Papoutsopoulou, S. Sortilin Expression Levels and Peripheral Immunity: A Potential Biomarker for Segregation between Parkinson’s Disease Patients and Healthy Controls. Int. J. Mol. Sci. 2024, 25, 1791. https://doi.org/10.3390/ijms25031791
Georgoula M, Ntavaroukas P, Androutsopoulou A, Xiromerisiou G, Kalala F, Speletas M, Asprodini E, Vasilaki A, Papoutsopoulou S. Sortilin Expression Levels and Peripheral Immunity: A Potential Biomarker for Segregation between Parkinson’s Disease Patients and Healthy Controls. International Journal of Molecular Sciences. 2024; 25(3):1791. https://doi.org/10.3390/ijms25031791
Chicago/Turabian StyleGeorgoula, Maria, Panagiotis Ntavaroukas, Anastasia Androutsopoulou, Georgia Xiromerisiou, Fani Kalala, Matthaios Speletas, Eftihia Asprodini, Anna Vasilaki, and Stamatia Papoutsopoulou. 2024. "Sortilin Expression Levels and Peripheral Immunity: A Potential Biomarker for Segregation between Parkinson’s Disease Patients and Healthy Controls" International Journal of Molecular Sciences 25, no. 3: 1791. https://doi.org/10.3390/ijms25031791
APA StyleGeorgoula, M., Ntavaroukas, P., Androutsopoulou, A., Xiromerisiou, G., Kalala, F., Speletas, M., Asprodini, E., Vasilaki, A., & Papoutsopoulou, S. (2024). Sortilin Expression Levels and Peripheral Immunity: A Potential Biomarker for Segregation between Parkinson’s Disease Patients and Healthy Controls. International Journal of Molecular Sciences, 25(3), 1791. https://doi.org/10.3390/ijms25031791