Clinically Effective Molecules of Natural Origin for Obesity Prevention or Treatment
Abstract
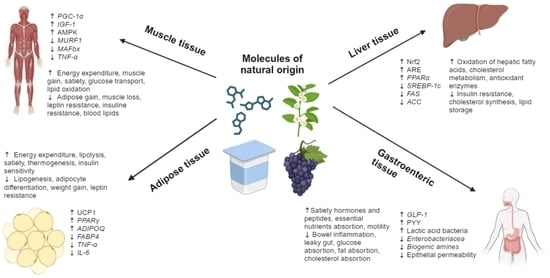
:1. Introduction
2. Etiopathogenic Mechanisms of Obesity
2.1. Genetic Factors
2.2. Epigenetic Factors
2.3. Energy Imbalance
2.4. Neuroendocrine Dysregulation
2.5. Neurophysiological Factors
2.6. Gut Microbiota
2.7. Other Etiopathogenic Factors
3. Molecules with Demonstrated Clinical Efficacy in the Prevention or Treatment of Obesity
3.1. Epigallocatechin-3-Gallate
3.2. Ellagic Acid
3.3. Resveratrol
3.4. Berberine
3.5. Anthocyanins
3.6. Probiotics
3.7. Carotenoids
3.8. Curcumin
3.9. Silymarin/Silybin
3.10. Hydroxycitric Acid
3.11. α-Lipoic Acid
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tutor, A.W.; Lavie, C.J.; Kachur, S.; Milani, R.V.; Ventura, H.O. Updates on obesity and the obesity paradox in cardiovascular diseases. Prog. Cardiovasc. Dis. 2023, 78, 2. [Google Scholar] [CrossRef]
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef]
- McPherson, R. Obesity and ischemic heart disease. Circ. Res. 2015, 116, 570. [Google Scholar] [CrossRef]
- Shiozawa, M.; Kaneko, H.; Itoh, H.; Morita, K.; Okada, A.; Matsuoka, S.; Kiriyama, H.; Kamon, T.; Fujiu, K.; Michihata, N.; et al. Association of body mass index with ischemic and hemorrhagic stroke. Nutrients 2021, 13, 2343. [Google Scholar] [CrossRef]
- Smith, K.B.; Smith, M.S. Obesity statistics. Prim. Care 2016, 43, 121. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.J.F.; Yeo, G.S.H. The genetics of obesity: From discovery to biology. Nat. Rev. Genet. 2022, 23, 120. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288. [Google Scholar] [CrossRef] [PubMed]
- Kaufer-Horwitz, M.; Pérez-Hernández, J.F.; Kaufer-Horwitz, M.; Pérez-Hernández, J.F. La obesidad: Aspectos fisiopatológicos y clínicos. Inter. Discip. 2022, 10, 147. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, B.; Wu, B.; Xiao, H.; Li, Z.; Li, R.; Xu, X.; Li, T. Signaling pathways in obesity: Mechanisms and therapeutic interventions. Sig. Transduct. Target Ther. 2022, 7, 1. [Google Scholar] [CrossRef]
- Grarup, N.; Moltke, I.; Andersen, M.K.; Dalby, M.; Vitting-Seerup, K.; Kern, T.; Mahendran, Y.; Jørsboe, E.; Larsen, C.V.L.; Dahl-Petersen, I.K.; et al. Loss-of-function variants in ADCY3 increase risk of obesity and type 2 diabetes. Nat. Genet. 2018, 50, 172. [Google Scholar] [CrossRef]
- Yin, D.; Li, Y.; Liao, X.; Tian, D.; Xu, Y.; Zhou, C.; Liu, J.; Li, S.; Zhou, J.; Nie, Y.; et al. FTO: A critical role in obesity and obesity-related diseases. Br. J. Nutr. 2023, 130, 1657–1664. [Google Scholar] [CrossRef]
- Wu, O.; Yuan, C.; Leng, J.; Zhang, X.; Liu, W.; Yang, F. Colorable role of interleukin (IL)-6 in obesity hypertension: A hint from a Chinese adult case-control study. Cytokine 2023, 168, 156226. [Google Scholar] [CrossRef] [PubMed]
- Bombarda-Rocha, V.; Silva, D.; Badr-Eddine, A.; Nogueira, P.; Gonçalves, J.; Fresco, P. Challenges in pharmacological intervention in perilipins (PLINs) to modulate lipid droplet dynamics in obesity and cancer. Cancers 2023, 15, 4013. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, N.; Auger, K.; Rahimi, N.; Jialal, I. Biochemistry, Adiponectin; StatPearls Publishing: St. Petersburg, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK537041/ (accessed on 29 September 2023).
- Ayed, K.; Nabi, L.; Akrout, R.; Mrizak, H.; Gorrab, A.; Bacha, D.; Boussen, H.; Gati, A. Obesity and cancer: Focus on leptin. Mol. Biol. Rep. 2023, 50, 6177. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, R.; Hu, C. Leptin/obR signaling exacerbates obesity-related neutrophilic airway inflammation through inflammatory M1 macrophages. Mol. Med. 2023, 29, 100. [Google Scholar] [CrossRef]
- Lechner, L.; Opitz, R.; Silver, M.J.; Krabusch, P.M.; Prentice, A.M.; Field, M.S.; Stachelscheid, H.; Leitão, E.; Schröder, C.; Fernandez Vallone, V.; et al. Early-set POMC methylation variability is accompanied by increased risk for obesity and is addressable by MC4R agonist treatment. Sci. Transl. Med. 2023, 15, 1659. [Google Scholar] [CrossRef] [PubMed]
- Shaji, A.; Jayasri, M.A. A review of the role of liposome-encapsulated phytochemicals targeting PPAR Ɣ and associated pathways to combat obesity. 3 Biotech 2023, 13, 313. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, H.Y.; Kim, H.J.; Lee, G.H.; Lim, Y.J.; Ko, B.M.; Kim, J.H.; Kim, T.W.; Kim, H.K.; Kim, T.Y.; et al. Combined treatment of mori folium and mori cortex radicis ameliorate obesity in mice via UCP-1 in brown adipocytes. Nutrients 2023, 15, 3713. [Google Scholar] [CrossRef]
- Rohde, K.; Keller, M.; la Cour-Poulsen, L.; Blüher, M.; Kovacs, P.; Böttcher, Y. Genetics and epigenetics in obesity. Metabolism 2019, 92, 37. [Google Scholar] [CrossRef]
- Ouni, M.; Schürmann, A. Epigenetic contribution to obesity. Mamm. Genom. 2020, 31, 134. [Google Scholar] [CrossRef]
- Pagiatakis, C.; Musolino, E.; Gornati, R.; Bernardini, G.; Papait, R. Epigenetics of aging and disease: A brief overview. Aging Clin. Exp. Res. 2021, 33, 737. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Izquierdo, D.; Torres-Martos, Á.; Baig, A.T.; Aguilera, C.M.; Ruiz-Ojeda, F.J. Impact of physical activity and exercise on the epigenome in skeletal muscle and effects on systemic metabolism. Biomedicines 2022, 10, 126. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Q.; Chang, C. Epigenetics in Health and Disease. In Epigenetics in Allergy and Autoimmunity; Chang, C., Lu, Q., Eds.; Springer: Singapore, 2020; pp. 3–55. [Google Scholar] [CrossRef]
- Wu, F.Y.; Yin, R.X. Recent progress in epigenetics of obesity. Diabetol. Metab. Syndr. 2022, 14, 171. [Google Scholar] [CrossRef]
- Şanlı, E.; Kabaran, S. Maternal obesity, maternal overnutrition and fetal programming: Effects of epigenetic mechanisms on the development of metabolic disorders. Curr. Genom. 2019, 20, 419. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Rönn, T. Epigenetics in human obesity and type 2 diabetes. Cell Metab. 2019, 29, 1028. [Google Scholar] [CrossRef] [PubMed]
- Grijalva-Avila, J.; Villanueva-Fierro, I.; Lares-Asseff, I.; Chairez-Hernández, I.; Rivera-Sanchez, G.; Martínez-Estrada, S.; Martínez-Rivera, I.; Quiñones, L.A.; Loera-Castañeda, V. Milk intake and IGF-1 rs6214 polymorphism as protective factors to obesity. Int. J. Food Sci. Nutr. 2020, 71, 388. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.; Fadadu, R.P.; Gaskins, A.J.; Rifas-Shiman, S.L.; Laue, H.E.; Moley, K.H.; Hivert, M.F.; Baccarelli, A.; Oken, E.; Chavarro, J.E.; et al. Dietary fat intake during early pregnancy is associated with cord blood DNA methylation at IGF2 and H19 genes in newborns. Environ. Mol. Mutagen. 2021, 62, 388. [Google Scholar] [CrossRef]
- Kim, J.Y.; Mondaca-Ruff, D.; Singh, S.; Wang, Y. SIRT1 and autophagy: Implications in endocrine disorders. Front. Endocrinol. 2022, 13, 930919. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Bedolla, M.; Barajas, A.; Cortés, F.; Alonso, D.; Hernández, A.; Ostoa, Z.; Salguero, I. Papel pleiotrópico y homeostático de las sirtuinas en la función biológica humana. Cienc. Huasteca Boletín Científico Esc. Super. Huejutla 2020, 8, 6. [Google Scholar] [CrossRef]
- Lahsen, M.R.; Kuzmanic, V.A. Cirugía metabólica 10 años después: Una mirada desde la diabetología. Rev. Med. Clin. Condes. 2016, 27, 188. [Google Scholar] [CrossRef]
- Nicoletti, C.F.; Cortes-Oliveira, C.; Pinhel, M.A.S.; Nonino, C.B. Bariatric surgery and precision nutrition. Nutrients 2017, 9, 974. [Google Scholar] [CrossRef]
- Hunter, D.J.; James, L.S.; Hussey, B.; Ferguson, R.A.; Lindley, M.R.; Mastana, S.S. Impacts of eccentric resistance exercise on DNA methylation of candidate genes for inflammatory cytokines in skeletal muscle and leukocytes of healthy males. Genes 2023, 14, 478. [Google Scholar] [CrossRef] [PubMed]
- Milton, K.; Gomersall, S.R.; Schipperijn, J. Let’s get moving: The Global Status Report on Physical Activity 2022 calls for urgent action. J. Sport Health Sci. 2023, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Siegel, R.L.; Torre, L.A.; Pearson-Stuttard, J.; Islami, F.; Fedewa, S.A.; Goding Sauer, A.; Shuval, K.; Gapstur, S.M.; Jacobs, E.J.; et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J. Clin. 2019, 69, 88. [Google Scholar] [CrossRef] [PubMed]
- González-Jiménez, E. Obesidad: Análisis etiopatogénico y fisiopatológico. Endocrinol. Nutr. 2013, 60, 17. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Sinha, S. Obesity and aging: Molecular mechanisms and therapeutic approaches. Ageing Res. Rev. 2021, 67, 101268. [Google Scholar] [CrossRef] [PubMed]
- Hafidi, M.E.; Buelna-Chontal, M.; Sánchez-Muñoz, F.; Carbó, R. Adipogenesis: A necessary but harmful strategy. Int. J. Mol. Sci. 2019, 20, 3657. [Google Scholar] [CrossRef]
- Zolla, L. On the need to distinguish between insulin-normal and insulin-resistant patients in testosterone therapy. Int. J. Mol. Sci. 2022, 23, 12730. [Google Scholar] [CrossRef]
- Malfacini, D.; Pfeifer, A. GPCR in adipose tissue function-focus on lipolysis. Biomedicines 2023, 11, 588. [Google Scholar] [CrossRef]
- Lytle, K.A.; Bush, N.C.; Triay, J.M.; Kellogg, T.A.; Kendrick, M.L.; Swain, J.M.; Gathaiya, N.W.; Hames, K.C.; Jensen, M.D. Adipocyte proteins and storage of endogenous fatty acids in visceral and subcutaneous adipose tissue in severe obesity. Obesity 2021, 29, 1014. [Google Scholar] [CrossRef]
- Horwitz, A.; Birk, R. Adipose tissue hyperplasia and hypertrophy in common and syndromic obesity—The case of BBS obesity. Nutrients 2023, 15, 3445. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Efthymiou, V.; Kodani, S.D.; Shamsi, F.; Patti, M.E.; Tseng, Y.H.; Streets, A. Mapping the transcriptional landscape of human white and brown adipogenesis using single-nuclei RNA-seq. Mol. Metab. 2023, 74, 101746. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.O.; Joo, H.K.; Lee, Y.R.; Kim, S.; Lee, K.H.; Lee, S.D.; Jeon, B. APE1/Ref-1 inhibits adipogenic transcription factors during adipocyte differentiation in 3T3-L1 cells. Int. J. Mol. Sci. 2023, 24, 3251. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, X.; Tang, Q.Q. Transcriptional regulation of adipocyte differentiation: A central role for CCAAT/Enhancer-binding Protein (C/EBP) β. J. Biol. Chem. 2015, 290, 755. [Google Scholar] [CrossRef] [PubMed]
- Kahn, C.R.; Wang, G.; Lee, K.Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Investig. 2019, 129, 3990. [Google Scholar] [CrossRef]
- Yang, R.; Barouch, L.A. Leptin signaling and obesity. Circ. Res. 2007, 101, 545. [Google Scholar] [CrossRef]
- Gjermeni, E.; Kirstein, A.S.; Kolbig, F.; Kirchhof, M.; Bundalian, L.; Katzmann, J.L.; Laufs, U.; Blüher, M.; Garten, A.; Le Duc, D. Obesity—An update on the basic pathophysiology and review of recent therapeutic advances. Biomolecules 2021, 11, 1426. [Google Scholar] [CrossRef]
- Gruzdeva, O.; Borodkina, D.; Uchasova, E.; Dyleva, Y.; Barbarash, O. Leptin resistance: Underlying mechanisms and diagnosis. Diabetes Metab. Syndr. Obes. 2019, 12, 191. [Google Scholar] [CrossRef]
- Achari, A.E.; Jain, S.K. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef]
- Ruan, H.; Dong, L.Q. Adiponectin signaling and function in insulin target tissues. J. Mol. Cell Biol. 2016, 8, 101. [Google Scholar] [CrossRef]
- Kalra, S.; Chawla, K.L.; Kapoor, N. Motivation and obesity care. J. Pak. Med. Assoc. 2023, 74, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Lopera, D.T.; Restrepo, M. Aspectos psicológicos de la obesidad en adultos. Rev. Psicol. Univ. Antioq. 2014, 6, 91. [Google Scholar]
- Sarwer, D.B.; Polonsky, H.M. The psychosocial burden of obesity. Endocrinol. Metab. Clin. N. Am. 2016, 45, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Sebert, C.; Kösling, C.; Grünwald, M.; Hilbert, A.; Hübner, C.; Schäfer, L. Neuropsychological and Neurophysiological Indicators of General and Food-Specific Impulsivity in Children with Overweight and Obesity: A Pilot Study. Nutrients 2018, 10, 1983. [Google Scholar] [CrossRef]
- Cohen, D.A. Neurophysiological pathways to obesity: Below awareness and beyond individual control. Diabetes 2008, 57, 1768–1773. [Google Scholar] [CrossRef]
- López-Espinoza, A.; Moreno, M. La Educación en Alimentación y Nutrición, 1st ed.; McGraw-Hill/Interamericana Editores: New York, NY, USA, 2016; ISBN 978-607-15-1371-7. [Google Scholar]
- Puljiz, Z.; Kumric, M.; Vrdoljak, J.; Martinovic, D.; Ticinovic, K.T.; Krnic, M.O. Obesity, gut microbiota, and metabolome: From pathophysiology to nutritional interventions. Nutrients 2023, 15, 2236. [Google Scholar] [CrossRef]
- Lee, J.Y.; Tsolis, R.M.; Bäumler, A.J. The microbiome and gut homeostasis. Science 2022, 377, eabp9960. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, L.; Yang, L.; Chu, H. The critical role of gut microbiota in obesity. Front. Endocrinol. 2022, 13, 1025706. [Google Scholar] [CrossRef]
- Fontané, L.; Benaiges, D.; Goday, A.; Llauradó, G.; Pedro-Botet, J. Influencia de la microbiota y de los probióticos en la obesidad. Clin. Investig. Arterioscler. 2018, 30, 271. [Google Scholar] [CrossRef]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial dysbiosis-induced obesity: Role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr. 2020, 123, 1127. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J. The first microbial colonizers of the human gut: Composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [PubMed]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current understanding of dysbiosis in disease in human and animal models. Inflamm. Bowel Dis. 2016, 22, 1137. [Google Scholar] [CrossRef] [PubMed]
- Cardinelli, C.S.; Sala, P.C.; Alves, C.C.; Torrinhas, R.S.; Waitzberg, D.L. Influence of intestinal microbiota on body weight gain: A narrative review of the literature. Obes. Surg. 2015, 25, 346. [Google Scholar] [CrossRef] [PubMed]
- Morales-Franco, B.; Nava-Villalba, M.; Medina-Guerrero, E.O.; Sánchez-Nuño, Y.A.; Davila-Villa, P.; Anaya-Ambriz, E.J. Host-pathogen molecular factors contribute to the pathogenesis of Rhizopus spp. in diabetes mellitus. Curr. Trop. Med. Rep. 2021, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.N.; Liu, X.T.; Liang, Z.H.; Wang, J.H. Gut microbiota in obesity. World J. Gastroenterol. 2021, 27, 3837. [Google Scholar] [CrossRef] [PubMed]
- Waters, J.L.; Ley, R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Vitale, R.M.; Iannotti, F.A.; Silvestri, C.; Flamand, N.; Druart, C.; Everard, A.; Pelicaen, R.; Maiter, D.; Thissen, J.P.; et al. Beneficial Effects of Akkermansia muciniphila Are Not Associated with Major Changes in the Circulating Endocannabinoidome but Linked to Higher Mono-Palmitoyl-Glycerol Levels as New PPARα Agonists. Cells 2021, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Crovesy, L.; Ostrowski, M.; Ferreira, D.M.T.P.; Rosado, E.L.; Soares-Mota, M. Effect of Lactobacillus on body weight and body fat in overweight subjects: A systematic review of randomized controlled clinical trials. Int. J. Obes. 2017, 41, 1607. [Google Scholar] [CrossRef]
- Moore, S.C.; Matthews, C.E.; Sampson, J.N.; Stolzenberg-Solomon, R.Z.; Zheng, W.; Cai, Q. Human metabolic correlates of body mass index. Metabolomics 2014, 10, 259. [Google Scholar] [CrossRef]
- Miller, G.D. Appetite regulation: Hormones, peptides, and neurotransmitters and their role in obesity. Am. J. Lifestyle Med. 2019, 13, 586. [Google Scholar] [CrossRef]
- Albrecht, U. The circadian clock, metabolism and obesity. Obes. Rev. 2017, 18, 25. [Google Scholar] [CrossRef]
- Marjani, A.; Khatami, A.; Saadati, H.; Asghari, M.; Razizadeh, M.H.; Abbasi, A. Association of adenovirus 36 infection and obesity: An updated meta-analysis of community-based studies. Rev. Med. Virol. 2022, 32, e2255. [Google Scholar] [CrossRef]
- Calloway, E.E.; Parks, C.A.; Bowen, D.J.; Yaroch, A.L. Environmental, social, and economic factors related to the intersection of food security, dietary quality, and obesity: An introduction to a special issue of the Translational Behavioral Medicine journal. Transl. Behav. Med. 2019, 9, 823. [Google Scholar] [CrossRef]
- James, A.; Wang, K.; Wang, Y. Therapeutic activity of green tea epigallocatechin-3-gallate on metabolic diseases and non-alcoholic fatty liver diseases: The current updates. Nutrients 2023, 15, 3022. [Google Scholar] [CrossRef]
- Andreu-Fernández, V.; Almeida-Toledano, L.; Pizarro-Lozano, N.; Navarro-Tapia, E.; Gómez-Roig, M.D.; De la Torre-Fornell, R. Bioavailability of epigallocatechin gallate administered with different nutritional strategies in healthy volunteers. Antioxidants 2020, 9, 440. [Google Scholar] [CrossRef]
- Van-Amelsvoort, J.M.; Van-Hof, K.H.; Mathot, J.N.; Mulder, T.P.; Wiersma, A.; Tijburg, L.B. Plasma concentrations of individual tea catechins after a single oral dose in humans. Xenobiotica 2001, 31, 891. [Google Scholar] [CrossRef]
- Yuan, H.; Li, Y.; Ling, F.; Guan, Y.; Zhang, D.; Zhu, Q. The phytochemical epigallocatechin gallate prolongs the lifespan by improving lipid metabolism, reducing inflammation and oxidative stress in high-fat diet-fed obese rats. Aging Cell 2020, 19, e13199. [Google Scholar] [CrossRef] [PubMed]
- Savova, M.S.; Mihaylova, L.V.; Tews, D.; Wabitsch, M.; Georgiev, M.I. Targeting PI3K/AKT signaling pathway in obesity. Biomed. Pharmacother. 2023, 159, 114244. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, R.; Yamamoto, M.; Kumazoe, M.; Fujimura, Y.; Yonekura, M.; Shimamoto, Y. The combined effect of green tea and α-glucosyl hesperidin in preventing obesity: A randomized placebo-controlled clinical trial. Sci. Rep. 2021, 11, 19067. [Google Scholar] [CrossRef]
- Chatree, S.; Sitticharoon, C.; Maikaew, P.; Pongwattanapakin, K.; Keadkraichaiwat, I.; Churintaraphan, M. Epigallocatechin gallate decreases plasma triglyceride, blood pressure, and serum kisspeptin in obese human subjects. Exp. Biol. Med. 2021, 246, 163. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Giner, R.M.; Marín, M.; Recio, M.C. A pharmacological update of ellagic acid. Planta Med. 2018, 84, 1068. [Google Scholar] [CrossRef]
- Clifford, M.N.; Scalbert, A. Ellagitannins—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1118. [Google Scholar] [CrossRef]
- Lu, G.; Wang, X.; Cheng, M.; Wang, S.; Ma, K. The multifaceted mechanisms of ellagic acid in the treatment of tumors: State-of-the-art. Biomed. Pharmacother. 2023, 165, 115132. [Google Scholar] [CrossRef]
- Kowshik, J.; Giri, H.; Kishore, T.K.K.; Kesavan, R.; Vankudavath, R.N.; Reddy, G.B. Ellagic acid inhibits VEGF/VEGFR2, PI3K/Akt and MAPK signaling cascades in the hamster cheek pouch carcinogenesis model. Anti-Cancer Agents Med. Chem. 2014, 14, 1249. [Google Scholar] [CrossRef] [PubMed]
- Polce, S.A.; Burke, C.; França, L.M.; Kramer, B.; de Andrade-Paes, A.M.; Carrillo-Sepulveda, M.A. Ellagic acid alleviates hepatic oxidative stress and insulin resistance in diabetic female rats. Nutrients 2018, 10, F531. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.S.; Choi, H.S.; Seo, M.J.; Jeon, H.J.; Lee, B.Y. Ellagic acid suppresses lipid accumulation by suppressing early adipogenic events and cell cycle arrest. Phytother. Res. 2015, 29, 398. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, L.; Ran, X.; Long, M.; Zhang, M.; Tao, Y. Ellagic acid reduces adipogenesis through inhibition of differentiation-prevention of the induction of rb phosphorylation in 3T3-L1 adipocytes. Evid. Based Complement Altern. Med. 2013, 2013, 287534. [Google Scholar] [CrossRef] [PubMed]
- Hudak, C.S.; Sul, H.S. Pref-1, a gatekeeper of adipogenesis. Front. Endocrinol. 2013, 4, 79. [Google Scholar] [CrossRef]
- Hidalgo-Lozada, G.M.; Villarruel-López, A.; Martínez-Abundis, E.; Vázquez-Paulino, O.; González-Ortiz, M.; Pérez-Rubio, K.G. Ellagic acid effect on the components of metabolic syndrome, insulin sensitivity and insulin secretion: A randomized, double-blind, placebo-controlled clinical trial. J. Clin. Med. 2022, 11, 5741. [Google Scholar] [CrossRef]
- Shiojima, Y.; Takahashi, M.; Kikuchi, M.; Akanuma, M. Effect of ellagic acid on body fat and triglyceride reduction in healthy overweight volunteers: A randomized, double-blind, placebo-controlled parallel group study. Funct. Foods Health Dis. 2020, 10, 180. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, S.; Wang, F.; Yu, H.; Li, X.; Dong, W. Chronic administration of ellagic acid improved the cognition in middle-aged overweight men. Appl. Physiol. Nutr. Metab. 2018, 43, 266. [Google Scholar] [CrossRef]
- Pasanta, D.; Htun, K.T.; Pan, J.; Tungjai, M.; Kaewjaeng, S.; Chancharunee, S. Waist Circumference and BMI are strongly correlated with mri-derived fat compartments in young adults. Life 2021, 11, 643. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J. Obesity and cardiovascular disease: A scientific statement from the american heart association. Circulation 2021, 143, e984. [Google Scholar] [CrossRef]
- Mohammadi, H.; Ohm, J.; Discacciati, A.; Sundstrom, J.; Hambraeus, K.; Jernberg, T. Abdominal obesity and the risk of recurrent atherosclerotic cardiovascular disease after myocardial infarction. Eur. J. Prev. Cardiol. 2020, 27, 1944. [Google Scholar] [CrossRef]
- Panchal, S.K.; Ward, L.; Brown, L. Ellagic acid attenuates high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. Eur. J. Nutr. 2013, 52, 559. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wei, Y.; Ning, C.; Zhang, M.; Fan, P.; Lei, D. Ellagic acid promotes browning of white adipose tissues in high-fat diet-induced obesity in rats through suppressing white adipocyte maintaining genes. Endocr. J. 2019, 66, 923. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, M.; Umemura, T.; Maeda, M.; Ishii, Y.; Okamura, T.; Inoue, T. Safety assessment of ellagic acid, a food additive, in a subchronic toxicity study using F344 rats. Food Chem. Toxicol. 2008, 46, 1119. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Randeva, H.S.; Pfeiffer, A.F.H.; Weickert, M.O. Implications of Resveratrol in Obesity and Insulin Resistance: A State-of-the-Art Review. Nutrients 2022, 14, 2870. [Google Scholar] [CrossRef]
- Diaz-Gerevini, G.T.; Repossi, G.; Dain, A.; Tarres, M.C.; Das, U.N.; Eynard, A.R. Beneficial action of resveratrol: How and why? Nutrition 2016, 32, 174. [Google Scholar] [CrossRef]
- Zhang, M.; Xue, Y.; Chen, H.; Meng, L.; Chen, B.; Gong, H. Resveratrol inhibits MMP3 and MMP9 expression and secretion by suppressing TLR4/NF-κB/STAT3 activation in Ox-LDL-treated HUVECs. Oxid. Med. Cell Longev. 2019, 2019, 9013169. [Google Scholar] [CrossRef]
- Yang, C.M.; Chen, Y.W.; Chi, P.L.; Lin, C.C.; Hsiao, L.D. Resveratrol inhibits BK-induced COX-2 transcription by suppressing acetylation of AP-1 and NF-κB in human rheumatoid arthritis synovial fibroblasts. Biochem. Pharmacol. 2017, 132, 77. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377. [Google Scholar] [CrossRef]
- Springer, M.; Moco, S. Resveratrol and Its Human Metabolites—Effects on Metabolic Health and Obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Hoca, M.; Becer, E.; Vatansever, H.S. The role of resveratrol in diabetes and obesity associated with insulin resistance. Arch. Physiol. Biochem. 2023, 129, 555. [Google Scholar] [CrossRef] [PubMed]
- Shahcheraghi, S.H.; Salemi, F.; Small, S.; Syed, S.; Salari, F.; Alam, W. Resveratrol regulates inflammation and improves oxidative stress via Nrf2 signaling pathway: Therapeutic and biotechnological prospects. Phytother. Res. 2023, 37, 1590. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, N.; Zhang, Z.; Li, W.; Zhu, W. Resveratrol induces cell apoptosis in adipocytes via AMPK activation. Biochem. Biophys. Res. Commun. 2015, 457, 608. [Google Scholar] [CrossRef] [PubMed]
- Asghari, S.; Asghari-Jafarabadi, M.; Somi, M.H.; Ghavami, S.M.; Rafraf, M. Comparison of Calorie-Restricted Diet and Resveratrol Supplementation on Anthropometric Indices, Metabolic Parameters, and Serum Sirtuin-1 Levels in Patients with Nonalcoholic Fatty Liver Disease: A Randomized Controlled Clinical Trial. J. Am. Coll. Nutr. 2018, 37, 223. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Tamtaji, O.R.; Lankarani, K.B.; Akbari, M.; Dadgostar, E.; Dabbaghmanesh, M.H. The effects of resveratrol intake on weight loss: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 375. [Google Scholar] [CrossRef] [PubMed]
- Ali-Sangouni, A.; Abdollahi, S.; Mozaffari-Khosravi, H. Effect of resveratrol supplementation on hepatic steatosis and cardiovascular indices in overweight subjects with type 2 diabetes: A double-blind, randomized controlled trial. BMC Cardiovasc. Disord. 2022, 22, 212. [Google Scholar] [CrossRef]
- Gu, W.; Geng, J.; Zhao, H.; Li, X.; Song, G. Effects of resveratrol on metabolic indicators in patients with type 2 diabetes: A systematic review and meta-analysis. Int. J. Clin. Pract. 2022, 2022, 9734738. [Google Scholar] [CrossRef]
- Delpino, F.M.; Figueiredo, L.M.; Caputo, E.L.; Mintem, G.C.; Gigante, D.P. What is the effect of resveratrol on obesity? A systematic review and meta-analysis. Clin. Nutr. ESPEN 2021, 41, 59. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Milajerdi, A.; Sheikhi, A.; Kord-Varkaneh, H.; Feinle-Bisset, C.; Larijani, B.; Esmaillzadeh, A. Resveratrol supplementation significantly influences obesity measures: A systematic review and dose-response meta-analysis of randomized controlled trials. Obes. Rev. 2019, 20, 487. [Google Scholar] [CrossRef]
- Noh, J.W.; Jun, M.S.; Yang, H.K.; Lee, B.C. cellular and molecular mechanisms and effects of berberine on obesity-induced inflammation. Biomedicines 2022, 10, 1739. [Google Scholar] [CrossRef]
- Han, Y.; Xiang, Y.; Shi, Y.; Tang, X.; Pan, L.; Gao, J.; Bi, R.; Lai, X. Pharmacokinetics and pharmacological activities of berberine in diabetes mellitus treatment. Evid.-Based Complement. Altern. Med. 2021, 2021, 9987097. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Davies, G.E. Berberine inhibits adipogenesis in high-fat diet-induced obesity mice. Fitoterapia 2010, 81, 358. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Tsai, J.; Tan, G.; Dalgin, G.; Hotamisligil, G.S. Interaction between GATA and the C/EBP family of transcription factors is critical in GATA-mediated suppression of adipocyte differentiation. Mol. Cell Biol. 2005, 25, 706. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Dalgin, G.; Xu, H.; Ting, C.N.; Leiden, J.M.; Hotamisligil, G.S. Function of GATA transcription factors in preadipocyte-adipocyte transition. Science 2000, 290, 134. [Google Scholar] [CrossRef]
- Choi, B.H.; Ahn, I.S.; Kim, Y.H.; Park, J.W.; Lee, S.Y.; Hyun, C.K.; Do, M.-S. Berberine reduces the expression of adipogenic enzymes and inflammatory molecules of 3T3-L1 adipocyte. Exp. Mol. Med. 2006, 38, 599. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, W.S.; Kim, K.H.; Yoon, M.J.; Cho, H.J.; Shen, Y.; Ye, J.-M.; Lee, C.H.; Oh, W.K.; Kim, C.T.; et al. Berberine, a Natural Plant Product, Activates AMP-Activated Protein Kinase with Beneficial Metabolic Effects in Diabetic and Insulin-Resistant States. Diabetes 2006, 55, 2256. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, T.; Ma, G.; Zheng, L.; Jiang, X.; Yang, F.; Wang, Z.; Li, N.; He, Z.; Song, X.; et al. Berberine modulates deacetylation of PPARγ to promote adipose tissue remodeling and thermogenesis via AMPK/SIRT1 pathway. Int. J. Biol. Sci. 2021, 17, 3173. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ye, X.; Hua, Y.; Song, Y. Berberine alleviates adipose tissue fibrosis by inducing AMP-activated kinase signaling in high-fat diet-induced obese mice. Biomed. Pharmacother. 2018, 105, 105121. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yin, J.; Gao, H.; Xu, L.; Wang, Y.; Xu, L.; Li, M. Berberine Improves Insulin Sensitivity by Inhibiting Fat Store and Adjusting Adipokines Profile in Human Preadipocytes and Metabolic Syndrome Patients. Evid. Based Complement. Altern. Med. 2012, 2012, 363845. [Google Scholar] [CrossRef] [PubMed]
- Firouzi, S.; Malekahmadi, M.; Ghayour-Mobarhan, M.; Ferns, G.; Rahimi, H.R. Barberry in the treatment of obesity and metabolic syndrome: Possible mechanisms of action. Diabetes Metab. Syndr. Obes. 2018, 11, 699. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, Z.; Perna, S.; Al-Thawadi, S.; Alalwan, T.A.; Riva, A.; Petrangolini, G.; Gasparri, C.; Infantino, V.; Peroni, G.; Rondanelli, M. The effect of Berberine on weight loss in order to prevent obesity: A systematic review. Biomed. Pharmacother. 2020, 127, 110137. [Google Scholar] [CrossRef] [PubMed]
- Pei, C.; Zhang, Y.; Wang, P.; Zhang, B.; Fang, L.; Liu, B.; Meng, S. Berberine alleviates oxidized low-density lipoprotein-induced macrophage activation by downregulating galectin-3 via the NF-κB and AMPK signaling pathways. Phytother. Res. 2019, 33, 294. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Liu, J.; Tan, Y.; Feng, W.; Peng, C. Interactions between gut microbiota and berberine, a necessary procedure to understand the mechanisms of berberine. J. Pharm. Anal. 2022, 12, 541. [Google Scholar] [CrossRef] [PubMed]
- Xiong, P.; Niu, L.; Talaei, S.; Kord-Varkaneh, H.; Clark, C.C.T.; Găman, M.A. Rahmani, J.; Dorosti, M.; Mousavi, S.M.; Zarezadeh, M.; et al. The effect of berberine supplementation on obesity indices: A dose–response meta-analysis and systematic review of randomized controlled trials. Complement. Ther. Clin. Pract. 2020, 39, 101113. [Google Scholar] [CrossRef]
- Zhao, J.V.; Yeung, W.F.; Chan, Y.H.; Vackova, D.; Leung, J.Y.Y.; Ip, D.K.M. Zhao, J.; Ho, W.-K.; Tse, H.; Schooling, C.M. Effect of Berberine on Cardiovascular Disease Risk Factors: A Mechanistic Randomized Controlled Trial. Nutrients 2021, 13, 2250. [Google Scholar] [CrossRef]
- Amini, M.R.; Sheikhhossein, F.; Naghshi, S.; Djafari, F.; Askari, M.; Shahinfar, H.; Safabakhsh, M.; Jafari, A.; Shab-Bidar, S. Effects of berberine and barberry on anthropometric measures: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 49, 102337. [Google Scholar] [CrossRef]
- Rondanelli, M.; Gasparri, C.; Petrangolini, G.; Allegrini, P.; Avenoso, D.; Fazia, T.; Bernardinelli, L.; Peroni, G.; Patelli, Z.; Mansueto, F.; et al. Berberine phospholipid exerts a positive effect on the glycemic profile of overweight subjects with impaired fasting blood glucose (IFG): A randomized double-blind placebo-controlled clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 6718. [Google Scholar]
- Zhang, J.; Han, C.; Lu, W.Q.; Wang, N.; Wu, S.R.; Wang, Y.X.; Ma, J.P.; Wang, J.H.; Hao, C.; Yuan, D.H.; et al. randomized, multicenter and noninferiority study of amoxicillin plus berberine vs. tetracycline plus furazolidone in quadruple therapy for Helicobacter pylori rescue treatment. J. Dig. Dis. 2020, 21, 256. [Google Scholar] [CrossRef]
- Chen, Y.X.; Gao, Q.Y.; Zou, T.H.; Wang, B.M.; Liu, S.D.; Sheng, J.Q.; Ren, J.-L.; Zou, X.-P.; Liu, Z.-J.; Song, Y.-Y.; et al. Berberine versus placebo for the prevention of recurrence of colorectal adenoma: A multicentre, double-blinded, randomised controlled study. Lancet Gastroenterol. Hepatol. 2020, 5, 267. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zou, D.; Liu, W.; Yang, J.; Zhu, N.; Huo, L.; Wang, M.; Hong, J.; Wu, P.; et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J. Clin. Endocrinol. Metab. 2008, 93, 2559. [Google Scholar] [CrossRef]
- Jahnke, G.D.; Price, C.J.; Marr, M.C.; Myers, C.B.; George, J.D. Developmental toxicity evaluation of berberine in rats and mice. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2006, 77, 191. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Liu, X.; Wu, N.; Han, Y.; Wang, J.; Yu, Y.; Chen, Q. Efficacy and safety of berberine alone for several metabolic disorders: A systematic review and meta-analysis of randomized clinical trials. Front. Pharmacol. 2021, 12, 653887. [Google Scholar] [CrossRef] [PubMed]
- Santamarina, A.B.; Calder, P.C.; Estadella, D.; Pisani, L.P. Anthocyanins ameliorate obesity-associated metainflammation: Preclinical and clinical evidence. Nutr. Res. 2023, 114, 50. [Google Scholar] [CrossRef] [PubMed]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Rimac-Brnčić, S.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; de Pascual-Teresa, S. A review of factors affecting anthocyanin bioavailability: Possible implications for the inter-individual variability. Foods 2020, 9, 2. [Google Scholar] [CrossRef]
- Yoon, Y.; Yoon, H.; Park, H.M.; Song, S.; Yeum, K.J. Dietary anthocyanins against obesity and inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef]
- Rossi, A.; Serraino, I.; Dugo, P.; Di-Paola, R.; Mondello, L.; Genovese, T.; Morabito, D.; Dugo, G.; Sautebin, L.; Caputi, A.P.; et al. Protective effects of anthocyanins from blackberry in a rat model of acute lung inflammation. Free Radic. Res. 2003, 37, 891. [Google Scholar] [CrossRef]
- DeFuria, J.; Bennett, G.; Strissel, K.J.; Perfield, J.W.; Milbury, P.E.; Greenberg, A.S.; Obin, M.S. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J. Nutr. 2009, 139, 1510. [Google Scholar] [CrossRef]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A (13)C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.J.; Lee, C.Y. Strawberry and its anthocyanins reduce oxidative stress-induced apoptosis in PC12 cells. J. Agric. Food Chem. 2005, 53, 1984. [Google Scholar] [CrossRef] [PubMed]
- Ngamsamer, C.; Sirivarasai, J.; Sutjarit, N. The Benefits of Anthocyanins against Obesity-Induced Inflammation. Biomolecules 2022, 12, 852. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, X.; Zhang, J.; Lang, Y.Z.; Lu, L.; Mi, J.; Cao, Y.-L.; Yan, Y.-M.; Ran, L.-W. Preventive Effects of Anthocyanins from Lyciumruthenicum Murray in High-Fat Diet-Induced Obese Mice Are Related to the Regulation of Intestinal Microbiota and Inhibition of Pancreatic Lipase Activity. Molecules 2022, 27, 2141. [Google Scholar] [CrossRef] [PubMed]
- Fotschki, B.; Juśkiewicz, J.; Jurgoński, A.; Sójka, M. Fructo-Oligosaccharides and Pectins Enhance Beneficial Effects of Raspberry Polyphenols in Rats with Nonalcoholic Fatty Liver. Nutrients 2021, 13, 833. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Rodríguez-Werner, M.; Schlösser, A.; Winterhalter, P.; Rimbach, G. Fractionation, enzyme inhibitory and cellular antioxidant activity of bioactives from purple sweet potato (Ipomoea batatas). Food Chem. 2017, 221, 447. [Google Scholar] [CrossRef]
- Wu, T.; Gao, Y.; Guo, X.; Zhang, M.; Gong, L. Blackberry and Blueberry Anthocyanin Supplementation Counteract High-Fat-Diet-Induced Obesity by Alleviating Oxidative Stress and Inflammation and Accelerating Energy Expenditure. Oxid. Med. Cell Longev. 2018, 2018, 4051232. [Google Scholar] [CrossRef]
- Wu, T.; Yin, J.; Zhang, G.; Long, H.; Zheng, X. Mulberry and cherry anthocyanin consumption prevents oxidative stress and inflammation in diet-induced obese mice. Mol. Nutr. Food Res. 2016, 60, 687. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Tulipani, S.; Casoli, T.; Di-Stefano, G.; González-Paramás, A.M.; Santos-Buelga, C.; Busco, F.; Quiles, J.L.; Cordero, M.D.; et al. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014, 25, 289. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J. Nutr. 2015, 145, 742. [Google Scholar] [CrossRef]
- Arisi, T.O.P.; Gorski, F.; Eibel, B.; Barbosa, E.; Boll, L.; Waclawovsky, G.; Lehnen, A.M. Dietary intake of anthocyanins improves arterial stiffness, but not endothelial function, in volunteers with excess weight: A randomized clinical trial. Phytother. Res. 2023, 37, 798. [Google Scholar] [CrossRef]
- Park, S.; Choi, M.; Lee, M. Effects of anthocyanin supplementation on reduction of obesity criteria: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2021, 13, 2121. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Kounatidis, D.; Tsilingiris, D.; Panagopoulos, F.; Christodoulatos, G.S.; Evangelopoulos, A.; Karampela, I.; Dalamaga, M. The role of next-generation probiotics in obesity and obesity-associated disorders: Current knowledge and future perspectives. Int. J. Mol. Sci. 2023, 24, 6755. [Google Scholar] [CrossRef]
- Kobyliak, N.; Conte, C.; Cammarota, G.; Haley, A.P.; Styriak, I.; Gaspar, L.; Fusek, J.; Rodrigo, L.; Kruzliak, P. Probiotics in prevention and treatment of obesity: A critical view. Nutr. Metab. 2016, 13, 14. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, P.; Zhou, X.; Yuan, J.; Chen, Q. Probiotics therapy show significant improvement in obesity and neurobehavioral disorders symptoms. Front. Cell Infect. Microbiol. 2023, 13, 1178399. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Kadooka, Y.; Sato, M.; Imaizumi, K.; Ogawa, A.; Ikuyama, K.; Akai, Y.; Okano, M.; Kagoshima, M.; Tsuchida, T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010, 64, 636. [Google Scholar] [CrossRef] [PubMed]
- Uusitupa, H.M.; Rasinkangas, P.; Lehtinen, M.J.; Mäkelä, S.M.; Airaksinen, K.; Anglenius, H.; Ouwehand, A.C.; Maukonen, J. Bifidobacterium animalis subsp. lactis 420 for Metabolic Health: Review of the Research. Nutrients 2020, 12, 892. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; Gøbel, R.J.; Michaelsen, K.F.; Forssten, S.D.; Lahtinen, S.J.; Jakobsen, M. Effect of Lactobacillus salivarius Ls-33 on fecal microbiota in obese adolescents. Clin. Nutr. 2013, 32, 935. [Google Scholar] [CrossRef]
- Pedret, A.; Valls, R.M.; Calderón-Pérez, L.; Llauradó, E.; Companys, J.; Pla-Pagà, L.; Moragas, A.; Martín-Luján, F.; Ortega, Y.; Giralt, M.; et al. Effects of daily consumption of the probiotic Bifidobacterium animalis subsp. lactis CECT 8145 on anthropometric adiposity biomarkers in abdominally obese subjects: A randomized controlled trial. Int. J. Obes. 2019, 43, 1863. [Google Scholar] [CrossRef]
- Ben-Othman, R.; Ben-Amor, N.; Mahjoub, F.; Berriche, O.; El-Ghali, C.; Gamoudi, A.; Jamoussi, H. A clinical trial about effects of prebiotic and probiotic supplementation on weight loss, psychological profile and metabolic parameters in obese subjects. Endocrinol. Diabetes Metab. 2023, 6, e402. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, P.; Putri, R.R.; Marcus, C.; Hagman, E. Evaluating probiotic efficacy on weight loss in adults with overweight through a double-blind, placebo-controlled randomized trial. Sci. Rep. 2023, 13, 18200. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.S.Y.; Brunelle, L.; Pilon, G.; Cautela, B.G.; Tompkins, T.A.; Drapeau, V.; Marette, A.; Tremblay, A. Lacticaseibacillus rhamnosus HA-114 improves eating behaviors and mood-related factors in adults with overweight during weight loss: A randomized controlled trial. Nutr. Neurosci. 2023, 26, 667. [Google Scholar] [CrossRef] [PubMed]
- Mounien, L.; Tourniaire, F.; Landrier, J.F. Anti-obesity effect of carotenoids: Direct impact on adipose tissue and adipose tissue-driven indirect effects. Nutrients 2019, 11, 1562. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; Bonet, M.L.; Borel, P.; Keijer, J.; Landrier, J.F.; Milisav, I.; Ribot, J.; Riso, P.; Winklhofer-Roob, B.; Sharoni, Y.; et al. Mechanistic aspects of carotenoid health benefits—Where are we now? Nutr. Res. Rev. 2021, 34, 276. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, W.; Xiao, W. Astaxanthin: A promising therapeutic agent for organ fibrosis. Pharmacol. Res. 2023, 188, 106657. [Google Scholar] [CrossRef]
- Yang, G.; Liu, X.; Jing, X.; Wang, J.; Wang, H.; Chen, F.; Wang, W.; Shao, Y.; Cui, X. Astaxanthin suppresses oxidative stress and calcification in vertebral cartilage endplate via activating Nrf-2/HO-1 signaling pathway. Int. Immunopharmacol. 2023, 119, 110159. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, B.; Bai, Y.; Miao, T.; Rui, L.; Zhang, H.; Xia, B.; Li, Y.; Gao, S.; Wang, X.-D.; et al. Lycopene in protection against obesity and diabetes: A mechanistic review. Pharmacol. Res. 2020, 159, 104966. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Yan, S.; Guo, Y.; Wang, H.; Li, X.; Wang, L.; Hu, W.; Li, B.; Cui, W. The association between carotenoids and subjects with overweight or obesity: A systematic review and meta-analysis. Food Funct. 2021, 12, 4768. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Lu, J.; Kang, T.; Zhu, M.; Xiong, K.; Wang, J. Astaxanthin supplementation mildly reduced oxidative stress and inflammation biomarkers: A systematic review and meta-analysis of randomized controlled trials. Nutr. Res. 2022, 99, 40. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.Y.L.; Chan, S.M.N.; Tam, H.L.; Wong, E.S.W. Astaxanthin Influence on Health Outcomes of Adults at Risk of Metabolic Syndrome: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2050. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Chu, Y.; Lei, J.; Yao, Z.; Wang, P.; Chen, Z.; Wang, K.; Sang, X.; Han, X.; Wang, L.; et al. Pharmacological Mechanisms and Clinical Applications of Curcumin: Update. Aging Dis. 2023, 14, 716. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Y.; Chen, C.W.; Chen, L.K.; Chou, H.Y.; Chang, C.L.; Juan, C.C. Curcumin Attenuates Adipogenesis by Inducing Preadipocyte Apoptosis and Inhibiting Adipocyte Differentiation. Nutrients 2019, 11, 2307. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, X.B.; Ye, F.; Tian, W.X. Suppression of fatty acid synthase, differentiation and lipid accumulation in adipocytes by curcumin. Mol. Cell Biochem. 2011, 351, 19. [Google Scholar] [CrossRef]
- Baziar, N.; Parohan, M. The effects of curcumin supplementation on body mass index, body weight, and waist circumference in patients with nonalcoholic fatty liver disease: A systematic review and dose-response meta-analysis of randomized controlled trials. Phytother. Res. 2020, 34, 464. [Google Scholar] [CrossRef]
- Unhapipatpong, C.; Polruang, N.; Shantavasinkul, P.C.; Julanon, N.; Numthavaj, P.; Thakkinstian, A. The effect of curcumin supplementation on weight loss and anthropometric indices: An umbrella review and updated meta-analyses of randomized controlled trials. Am. J. Clin. Nutr. 2023, 117, 1005. [Google Scholar] [CrossRef]
- Hellmann, P.H.; Bagger, J.I.; Carlander, K.R.; Forman, J.; Chabanova, E.; Svenningsen, J.S.; Holst, J.J.; Gillum, M.P.; Vilsbøll, T.; Knop, F.K. The effect of curcumin on hepatic fat content in individuals with obesity. Diabetes Obes. Metab. 2022, 24, 2192. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; He, L.; Liu, L.; Cheng, B.; Zhou, F.; Cao, D.; He, Y. A Comprehensive Review on the Benefits and Problems of Curcumin with Respect to Human Health. Molecules 2022, 27, 4400. [Google Scholar] [CrossRef]
- Dance-Barnes, S.T.; Kock, N.D.; Moore, J.E.; Lin, E.Y.; Mosley, L.J.; D’Agostino, R.B., Jr.; McCoy, T.P.; Townsend, A.J.; Miller, M.S. Lung Tumor Promotion by Curcumin. Carcinogenesis 2009, 30, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Rithaporn, T.; Monga, M.; Rajasekaran, M. Curcumin: A potential vaginal contraceptive. Contraception 2003, 68, 219. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef]
- Hawke, R.L.; Schrieber, S.J.; Soule, T.A.; Wen, Z.; Smith, P.C.; Reddy, K.R.; Wahed, A.S.; Belle, S.H.; Afdhal, N.H.; Navarro, V.J.; et al. Silymarin Ascending Multiple Oral Dosing Phase I Study in Noncirrhotic Patients with Chronic Hepatitis, C.J. Clin. Pharmacol. 2010, 50, 434. [Google Scholar] [CrossRef]
- Zarrelli, A.; Romanucci, V.; Tuccillo, C.; Federico, A.; Loguercio, C.; Gravante, R.; Di Fabio, G. New silibinin glyco-conjugates: Synthesis and evaluation of antioxidant properties. Bioorg. Med. Chem. Lett. 2014, 24, 5147. [Google Scholar] [CrossRef]
- Saller, R.; Brignoli, R.; Melzer, J.; Meier, R. An updated systematic review with meta-analysis for the clinical evidence of silymarin. Forsch. Komplementmed. 2008, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Aghemo, A.; Alekseeva, O.P.; Angelico, F.; Bakulin, I.G.; Bakulina, N.V.; Bordin, D.; Bueverov, A.O.; Drapkina, O.M.; Gillessen, A.; Kagarmanova, E.M.; et al. Role of silymarin as antioxidant in clinical management of chronic liver diseases: A narrative review. Ann. Med. 2022, 54, 1548. [Google Scholar] [CrossRef]
- Kim, S.H.; Choo, G.S.; Yoo, E.S.; Woo, J.S.; Han, S.H.; Lee, J.H.; Jung, J.-Y. Silymarin induces inhibition of growth and apoptosis through modulation of the MAPK signaling pathway in AGS human gastric cancer cells. Oncol. Rep. 2019, 42, 1904. [Google Scholar] [CrossRef]
- Gao, X.; Xiao, Z.H.; Liu, M.; Zhang, N.Y.; Khalil, M.M.; Gu, C.Q.; Qi, D.-S.; Sun, L.-H. Dietary silymarin supplementation alleviates zearalenone-induced hepatotoxicity and reproductive toxicity in rats. J. Nutr. 2018, 148, 1209. [Google Scholar] [CrossRef]
- Lee, J.A.; Shin, M.R.; Choi, J.; Kim, M.; Park, H.J.; Roh, S.S. Co-treatments of gardeniae fructus and silymarin ameliorates excessive oxidative stress-driven liver fibrosis by regulation of hepatic Sirtuin1 activities using thioacetamide-induced mice model. Antioxidants 2022, 12, 97. [Google Scholar] [CrossRef]
- Yassin, N.Y.S.; AbouZid, S.F.; El-Kalaawy, A.M.; Ali, T.M.; Elesawy, B.H.; Ahmed, O.M. Tackling of renal carcinogenesis in wistar rats by silybum marianum total extract, silymarin, and silibinin via modulation of oxidative stress, apoptosis, Nrf2, PPARγ, NF-κB, and PI3K/Akt signaling pathways. Oxid. Med. Cell Longev. 2021, 2021, 7665169. [Google Scholar] [CrossRef]
- Du, Q.; Wu, X.; Ma, K.; Liu, W.; Liu, P.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Ikejima, T. Silibinin alleviates ferroptosis of rat islet β cell INS-1 induced by the treatment with palmitic acid and high glucose through enhancing PINK1/parkin-mediated mitophagy. Arch. Biochem. Biophys. 2023, 743, 109644. [Google Scholar] [CrossRef]
- Sohail, I.; Malkani, N.; Tahir, N.; Khalil, A.; Attar, R.; Mumtaz, S. Silymarin protects the liver from α-naphthylisothiocyanate-induced cholestasis by modulating the expression of genes involved in bile acid homeostasis. Cell Mol. Biol. 2022, 68, 208. [Google Scholar] [CrossRef]
- Bellavite, P.; Fazio, S.; Affuso, F. A descriptive review of the action mechanisms of berberine, quercetin and silymarin on insulin resistance/hyperinsulinemia and cardiovascular prevention. Molecules 2023, 28, 4491. [Google Scholar] [CrossRef]
- Kalopitas, G.; Antza, C.; Doundoulakis, I.; Siargkas, A.; Kouroumalis, E.; Germanidis, G.; Samara, M.; Chourdakis, M. Impact of silymarin in individuals with nonalcoholic fatty liver disease: A systematic review and meta-analysis. Nutrition 2021, 83, 111092. [Google Scholar] [CrossRef]
- Aller, R.; Izaola, O.; Gómez, S.; Tafur, C.; González, G.; Berroa, E.; Mora, N.; González, J.M.; de Luis, D.A. Effect of silymarin plus vitamin E in patients with non-alcoholic fatty liver disease. A randomized clinical pilot study. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3118. [Google Scholar] [PubMed]
- Patti, A.M.; Al-Rasadi, K.; Katsiki, N.; Banerjee, Y.; Nikolic, D.; Vanella, L.; Giglio, R.V.; Giannone, V.A.; Montalto, G.; Rizzo, M. Effect of a natural supplement containing curcuma longa, guggul, and chlorogenic acid in patients with metabolic syndrome. Angiology 2015, 66, 856. [Google Scholar] [CrossRef]
- Amini, M.R.; Rasaei, N.; Jalalzadeh, M.; Akhgarjand, C.; Hashemian, M.; Jalali, P.; Hekmatdoost, A. The effects of Garcinia cambogia (hydroxycitric acid) on lipid profile: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2023, 38, 1028–1043. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Ueno, M.; Ogino, S.; Kubo, K.; Nagata, J.; Takeuchi, M. High dose of Garcinia cambogia is effective in suppressing fat accumulation in developing male Zucker obese rats, but highly toxic to the testis. Food Chem. Toxicol. 2005, 43, 411. [Google Scholar] [CrossRef] [PubMed]
- Fassina, P.; Scherer-Adami, F.; Zani, V.T.; Kasper-Machado, I.C.; Garavaglia, J.; Quevedo-Grave, M.T.; Ramos, R.; Morelo Dal Bosco, S. El efecto de la Garcinia Cambogia como coadyuvante en el proceso de perdida de peso. Nutr. Hosp. 2015, 32, 2400. [Google Scholar] [CrossRef]
- Maia-Landim, A.; Ramírez, J.M.; Lancho, C.; Poblador, M.S.; Lancho, J.L. Long-term effects of Garcinia cambogia/Glucomannan on weight loss in people with obesity, PLIN4, FTO and Trp64Arg polymorphisms. BMC Complement. Altern. Med. 2018, 18, 26. [Google Scholar] [CrossRef]
- Zovi, A.; Langella, R.; Nisic, A.; Vitiello, A.; Musazzi, U.M. Liver injury and dietary supplements: Does hydroxycitric acid trigger hepatotoxicity? J. Integr. Med. 2022, 20, 473. [Google Scholar] [CrossRef] [PubMed]
- Vuppalanchi, R.; Bonkovsky, H.L.; Ahmad, J.; Barnhart, H.; Durazo, F.; Fontana, R.J.; Gu, J.; Khan, I.; Kleiner, D.E.; Koh, C.; et al. Garcinia cambogia, Either Alone or in Combination with Green Tea, Causes Moderate to Severe Liver Injury. Clin. Gastroenterol. Hepatol. 2022, 20, e1416. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.W.; Beah, Z.M.; Grube, B.; Riede, L. IQP-GC-101 reduces body weight and body fat mass: A randomized, double-blind, placebo-controlled study. Phytother. Res. 2014, 28, 1520. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Jeon, S.M.; Park, K.H.; Lee, W.S.; Jeong, T.S.; McGregor, R.A.; Choi, M.-S. Does Glycine max leaves or Garcinia Cambogia promote weight-loss or lower plasma cholesterol in overweight individuals: A randomized control trial. Nutr. J. 2011, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Opala, T.; Rzymski, P.; Pischel, I.; Wilczak, M.; Wozniak, J. Efficacy of 12 weeks supplementation of a botanical extract-based weight loss formula on body weight, body composition and blood chemistry in healthy, overweight subjects—A randomised double-blind placebo-controlled clinical trial. Eur. J. Med. Res. 2006, 11, 343. [Google Scholar] [PubMed]
- Pons, L. Ácido lipoico: Un debate cosmético. Offarm 2003, 22, 157. [Google Scholar]
- Salehi, B.; Berkay-Yılmaz, Y.; Antika, G.; Boyunegmez-Tumer, T.; Fawzi-Mahomoodally, M.; Lobine, D.; Akram, M.; Riaz, M.; Capanoglu, E.; Sharopov, F.; et al. Insights on the Use of α-Lipoic Acid for Therapeutic Purposes. Biomolecules 2019, 9, 356. [Google Scholar] [CrossRef] [PubMed]
- Capece, U.; Moffa, S.; Improta, I.; Di Giuseppe, G.; Nista, E.C.; Cefalo, C.M.A.; Cinti, F.; Pontecorvi, A.; Gasbarrini, A.; Giaccari, A.; et al. Alpha-Lipoic Acid and Glucose Metabolism: A Comprehensive Update on Biochemical and Therapeutic Features. Nutrients 2022, 15, 18. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, J.Y.; Namkoong, C.; Jang, P.G.; Ryu, J.W.; Song, H.S.; Yun, J.-Y.; Namgoong, I.-S.; Ha, J.; Park, I.-S.; et al. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat. Med. 2004, 10, 727. [Google Scholar] [CrossRef]
- Ishida, Y.; Ohara, T.; Okuno, Y.; Ito, T.; Hirota, Y.; Furukawa, K.; Ogawa, W.; Kasuga, M. α-Lipoic Acid and Insulin Autoimmune Syndrome. Diabetes Care 2007, 30, 2240. [Google Scholar] [CrossRef]
- Vigil, M.; Berkson, B.M.; Garcia, A.P. Adverse effects of high doses of intravenous alpha lipoic Acid on liver mitochondria. Glob. Adv. Health Med. 2014, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Kucukgoncu, S.; Zhou, E.; Lucas, K.B.; Tek, C. Alpha-lipoic acid (ALA) as a supplementation for weight loss: Results from a meta-analysis of randomized controlled trials. Obes. Rev. 2017, 18, 594. [Google Scholar] [CrossRef] [PubMed]
- Sztolsztener, K.; Hodun, K.; Chabowski, A. α-lipoic acid ameliorates inflammation state and oxidative stress by reducing the content of bioactive lipid derivatives in the left ventricle of rats fed a high-fat diet. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166440. [Google Scholar] [CrossRef] [PubMed]
- Tutunchi, H.; Arefhosseini, S.; Ebrahimi-Mameghani, M. Clinical effectiveness of α-lipoic acid, myo-inositol and propolis supplementation on metabolic profiles and liver function in obese patients with NAFLD: A randomized controlled clinical trial. Clin. Nutr. ESPEN 2023, 54, 412. [Google Scholar] [CrossRef]
- Mohammadshahi, M.; Zakizadeh, E.; Ahmadi-Angali, K.; Ravanbakhsh, M.; Helli, B. The synergic effects of alpha-lipoic acid supplementation and electrical isotonic contraction on anthropometric measurements and the serum levels of VEGF, NO, sirtuin-1, and PGC1-α in obese people undergoing a weight loss diet. Arch. Physiol. Biochem. 2022, 128, 1195–1201. [Google Scholar] [CrossRef]
- Nasiri, G.; Bastani, A.; Haji-Aghamohammadi, A.A.; Nooshabadi, M.R.; Shahmirzalou, P.; Haghighian, H.K. Effects of probiotic and alpha-lipoic acid supplements, separately or in combination on the anthropometric indicators and maintenance of weight in overweight individuals. Clin. Nutr. ESPEN 2021, 41, 242. [Google Scholar] [CrossRef]
| Compound | Type of Study | Dose | Targeted Population | Observed Effect Δ | p | Adverse Effects | Reference |
|---|---|---|---|---|---|---|---|
| Epigallocatechin-3-gallate | Randomized, double-blind, placebo-controlled clinical trial in 30 patients. | 150 mg/day orally, for 8 weeks. | Men and women who are overweight or obese. | Significant decrease in plasma triacylglycerides, systolic blood pressure, and diastolic blood pressure. | <0.05 in all parameters. | Unmentioned | Chatree et al., 2021 [83] |
| Epigallocatechin-3-gallate + α-glucosyl hesperidin | Clinical trial, randomized, placebo-controlled, double-blind, in parallel groups with 60 patients. | 146 mg of EGCG + 178 mg of α-glucosyl hesperidin/day, orally, for 12 weeks. | Healthy Japanese men and women between 30 and 75 years old. | Reduction in BMI, triacylglycerides, body fat, visceral fat, and LDL-c/HDL-c ratio. | <0.05 in all parameters. | None | Yoshitomi et al., 2021 [82] |
| Compound | Type of Study | Dose | Targeted Population | Observed Effect Δ | p | Adverse Effects | Reference |
|---|---|---|---|---|---|---|---|
| Ellagic acid | Double-blind randomized clinical trial with 32 patients. | 1000 mg/day 12 weeks | Men and women with metabolic syndrome | Decrease in waist circumference H −2.7 cm M −3.8 cm | 0.03 0.01 | None | Hidalgo, et al., 2022 [92] |
| Ellagic acid | Double-blind randomized clinical trial with 32 patients. | 3 mg/day 12 weeks | Overweight men and women | Decrease in waist circumference −0.7 cm | <0.01 | None | Shiojima, et al., 2020 [93] |
| Ellagic acid | Double-blind randomized clinical trial with 150 patients. | 50 mg/day 12 weeks | Overweight men | Decrease in waist circumference −1.5 cm | <0.01 | None | Liu, et al., 2018 [94] |
| Compound | Type of Study | Dose | Targeted Population | Observed Effect Δ | p | Adverse Effects | Reference |
|---|---|---|---|---|---|---|---|
| Resveratrol | Systematic review and meta-analysis of 36 RCTs. | 10 to 200 mg/day orally for 4–12 weeks. | Men and women with overweight or obesity, as well as comorbidities. | Decrease in body weight, BMI, fat mass, and waist circumference. Increase in lean mass. No significant changes in serum leptin and adiponectin levels. | 0.03, 0.01, 0.03, 0.001 and <0.001, respectively. | None | Tabrizi et al., 2020 [111] |
| Resveratrol | Controlled, randomized, double-blind clinical trial with 71 participants. | 1 g/day orally for 8 weeks. | Men and women with overweight and DM2. | It had no effect on hepatic steatosis or cardiovascular indices. | Not significant. | None | Ali-Sangouni et al., 2022 [112] |
| Resveratrol | Systematic review and meta-analysis of 19 RCTs with a total of 1151 patients. | >1 g/day orally for 4–12 weeks. | Men and women with DM2 and comorbidities such as overweight and obesity. | Reduction of fasting serum glucose and systolic and diastolic blood pressure. No significance in waist circumference, serum triacylglycerides, or HDL-c. | <0.00001, <0.00001, 0.95, 0.66 and 0.14, respectively. | None | Gu et al., 2022 [113] |
| Resveratrol | Systematic review and meta-analysis of 28 RCTs. | <500 mg/day orally for ≥3 months. | Men and women with obesity. | Reduction in body weight, BMI, and waist circumference. No effects on fat mass. | 0.02, 0.02, 0.009 and 0.16, respectively. | None | Mousavi et al., 2019 [115] |
| Compound | Type of Study | Dose | Targeted Population | Observed Effect Δ | p | Adverse Effects | Reference |
|---|---|---|---|---|---|---|---|
| Berberine | Double-blind randomized clinical trial with 49 patients. | 1100 mg/day for 8 weeks. | Men and women with impaired fasting glucose and overweight. | Decrease in fat mass −1.0 kg, visceral fat −93.2 g and waist circumference −1.42 cm. | <0.001 for the three variables | None | Rondanelli, et al., 2023 [133] |
| Berberine | Systematic review with meta-analysis of 9 clinical trials with a total of 378 individuals. | 1000 to 1500 mg/day for 12–24 weeks. | Men and women who are overweight or obese in addition to having T2DM or metabolic syndrome. | Decrease in body weight. Decrease in BMI—0.29 kg/m2 and waist circumference −1.78cm | 0.004 0.001 | Not mentioned. | Xiong, et al., 2020 [130] |
| Berberine | Systematic review and meta-analysis of 12 clinical trials with a total of 849 individuals. | 1000 to 1500 mg/day for 12–24 weeks. | Men and women who are overweight or obese in addition to having T2DM or metabolic syndrome. | No change in body weight or BMI. Decrease in waist/hip ratio—0.03 | <0.001 | Not mentioned. | Amini, et al., 2020 [132] |
| Compound | Type of Study | Dose | Targeted Population | Observed Effect Δ | p | Adverse Effects | Reference |
|---|---|---|---|---|---|---|---|
| Anthocyanins | Randomized, placebo-controlled clinical trial with 55 participants. | 200 g/day of açaí-juçara juice (293.6 mg) orally for 12 weeks. | Men and women who are overweight or obese. | Decrease in arterial stiffness (pulse wave speed) and peripheral vascular resistance. No changes in flow-mediated dilation (endothelial function). | 0.002, 0.005 and >0.05, respectively. | None. | Arisi et al., 2023 [155] |
| Anthocyanins | Systematic review and meta-analysis of 11 RCTs with a total of 833 patients. | 28.3–500 mg/day orally for 4–24 weeks. | Women and men who are overweight or obese. | Reduction in BMI and body weight. No significant changes in waist circumference. | 0.002, 0.04, and >0.05, respectively. | Not mentioned. | Park et al., 2021 [156] |
| Compound | Type of Study | Dose | Targeted Population | Observed Effect Δ | p | Adverse Effects | Reference |
|---|---|---|---|---|---|---|---|
| Probiotics | Clinical trial, randomized, controlled, and matched by age, sex, and BMI with 45 participants. | One tablet/day orally with Bifidobacterium longum, Lactobacillus helveticus, Lactococcus lactis and Streptococcus thermophilus, for 4 weeks. | Women and men with obesity. | Reduction in weight, BMI, and waist circumference. Decrease in fat mass. Increased muscle strength. Decrease in fasting blood glucose. | <0.05, 0.001, 0.004 and 0.02. | None | Ben Othman et al., 2023 [167] |
| Probiotics | Double-blind, randomized, placebo-controlled clinical trial with 81 participants. | One capsule/day orally with 13 million CFU/g of Bacillus subtilis (LMG P-32899) and Bacillus coagulans (LMG P-32921) for 12 weeks. | Overweight men and women between 18–45 years of age. | Reduction in body weight. No significant changes in BMI, waist circumference, blood pressure, or biomarkers. | 0.027 and >0.05. | None | Danielsson et al., 2023 [168] |
| Probiotics | Randomized, double-blind, placebo-controlled clinical trial with 152 participants. | Oral capsules with Lacticaseibacillus rhamnosus HA-114 for 12 weeks. | Overweight adult men and women. | Significant decrease in plasma insulin, HOMA-IR, LDL cholesterol, and triacylglycerides. No significant changes in body weight and BMI. | <0.05 and >0.05. | None | Choi et al., 2023 [169] |
| Compound | Type of Study | Dose | Targeted Population | Observed Effect Δ | p | Adverse Effects | Reference |
|---|---|---|---|---|---|---|---|
| Carotenoids | Systematic review and meta-analysis of 7 RCTs and 8 observational studies, with 28,944 participants. | 1.2–60 mg/day orally for 20 days to 16 weeks. | Men and women who are overweight or obese. | Insufficiency of serum carotenoids is a risk factor for overweight and obesity (OR = 1.73). Reduction in body weight, BMI, and waist circumference. | <0.001, <0.001, <0.001 and <0.001, respectively. | Not mentioned | Yao et al., 2021 [175] |
| Carotenoids | Systematic review and meta-analysis of 12 RCTs with a total of 380 participants. | Oral supplementation with astaxanthin in variable doses and periods. | Men and women with overweight, obesity, and/or DM2. | Significant reduction in blood malondialdehyde concentration and IL-6. Improvement of superoxide dismutase activity and reduction of serum isoprostane concentration. | <0.01, 0.02, <0.05 and >0.05. | Not mentioned | Ma et al., 2022 [176] |
| Carotenoids | Systematic review and meta-analysis of 7 RCTs with a total of 321 participants. | Oral supplementation of 0.16–20 mg/day for 8 weeks to 12 months. | Men and women with overweight or obesity and metabolic syndrome. | Significant reduction in LDL. No significant changes in BMI, fasting blood glucose, systolic and diastolic blood pressure, total cholesterol, HDL, or triacylglycerols. | <0.00001 and ≥0.05. | No serious adverse effects were reported. | Leung et al., 2022 [177] |
| Compound | Type of Study | Dose | Targeted Population | Observed Effect Δ | p | Adverse Effects | Reference |
|---|---|---|---|---|---|---|---|
| Curcumin | Systematic review and meta-analysis of 8 clinical trials with 520 individuals. | 70 to 3000 mg/day for 8 to 12 weeks | Overweight men and women with nonalcoholic fatty liver disease (NAFLD) | No change in body weight Decrease in BMI −0.34 kg/m2 and waist circumference −2.12 cm | <0.05 <0.01 | Not mentioned. | Baziar et al., 2019 [181] |
| Curcumin | Review and meta-analysis of 14 SRMAs with 39 RCTs with 8111 individuals. | 70 to 3000 mg/day orally for 8 to 12 weeks | Men and women with overweight or obesity, NAFLD, T2DM, PCOS, or MetS | Decreased body weight −0.59 kg BMI −0.24 kg/m2/ Waist circumference −1.32 cm | Not mentioned. | Unhapipatpong et al., 2023 [182] |
| Compound | Type of Study | Dose | Targeted Population | Observed Effect Δ | p | Adverse Effects | Reference |
|---|---|---|---|---|---|---|---|
| Silymarin (Silybum marianum). | Systematic review and meta-analysis of 8 RCTs. | 100–500 mg/day orally for 4–12 weeks. | Men and women with NAFLD (many of them overweight or obese). | Statistically significant reduction in BMI. | <0.05. | None. | Kalopitas et al., 2021 [199] |
| Silymarin (Silybum marianum). | Clinical, randomized, and controlled trial with 36 participants. | Two tablets/day orally of silymarin + vitamin E for 12 weeks. | Men and women with NAFLD (many of them overweight or obese). | Decrease in body weight and anthropometric parameters | <0.05 and >0.05. | None. | Aller et al., 2015 [200] |
| Silymarin (Silybum marianum). | Clinical, randomized, and controlled trial with 78 participants. | Two tablets/day of oral supplement with Curcuma longa, silymarin, guggul, chlorogenic acid, and inulin for 16 weeks. | Men and women with metabolic syndrome (many of them overweight or obese). | Significant body weight, BMI, waist circumference, fasting glucose and total cholesterol reduction. | <0.0001, 0.001, 0.0004, 0.014, 0.03 and >0.05, respectively. | None. | Patti et al., 2015 [201] |
| Compound | Type of Study | Dose | Targeted Population | Observed Effect Δ | p | Adverse Effects | Reference |
|---|---|---|---|---|---|---|---|
| Hydroxycitric acid (G. cambogia). | Randomized, placebo-controlled, double-blind, parallel-group clinical trial with 91 participants. | Two tablets a day 30 min before meals for oral administration for 14 weeks. | Caucasian men and women who are overweight or obese. | Weight loss. Reduction of fat mass, waist circumference, and hip circumference. | 0.002 and <0.05. | There were no serious events reported. | Chong et al., 2014 [208] |
| Hydroxycitric acid (G. cambogia). | Clinical, randomized, and controlled trial with 86 participants. | Oral tablets at 2 g/day for 10 weeks. | Overweight men and women. | No significant effect on adipocytokines, non-HDL-c cholesterol, triacylglycerides, antioxidants, body weight, HDL-c, or total cholesterol. | >0.05. | Not mentioned. | Kim et al., 2011 [209] |
| Hydroxycitric acid (G. cambogia). | Randomized, double-blind, placebo-controlled clinical trial with 105 participants. | Polyherbal oral supplement in tablets with G. cambogia twice daily, for 12 weeks. | Men and women who are overweight or obese. | Significant change in the Body Composition Improvement Index. No significant changes in weight, BMI, and waist/hip ratio. Decrease in body fat. | 0.012, >0.05 and 0.011. | Not mentioned. | Opala et al., 2006 [210] |
| Hydroxycitric acid (G. cambogia). | Case series in 1418 patients enrolled in the Drug-Induced Liver Injury Network (DILIN) from 2004 to 2018. | Oral supplementation of varying doses of G. cambogia alone or in combination with green tea or Ashwagandha. | Men and women between 17 and 54 years old. | 22 cases of liver injury due to G. cambogia alone (n = 5) or in combination with green tea (n = 16) or Ashwagandha (n = 1), arising between 13 and 223 days after onset. Significant increase in aminotransferases. | <0.018 | Liver injury. Hepatocellular injury with jaundice. Hospitalization. Requirement of liver transplant. Death. | Vuppalanchi et al., 2022 [207] |
| Compound | Type of Study | Dose | Targeted Population | Observed Effect Δ | p | Adverse Effects | Reference |
|---|---|---|---|---|---|---|---|
| α-Lipoic acid | Clinical, controlled, and randomized trial with 92 participants | Oral administration for 8 weeks | Men and women with NAFLD and obesity | Reduction of alanine aminotransferase | 0.012 | Not mentioned | Tutunchi et al., 2023 [219] |
| α-Lipoic acid | Clinical, controlled, and randomized trial with 100 participants. | 1200 mg/day orally for 8 weeks. | Men and women with obesity | Reduction in weight, BMI, body fat, and waist circumference | <0.05 | Not mentioned | Mohammadshahi et al., 2022 [220] |
| α-Lipoic acid | Clinical, controlled, and randomized trial with 88 participants | 600 mg/day orally for 16 weeks | Overweight women and men | Reduction in weight, waist circumference, and C-reactive protein. Maintenance of lost weight | <0.05 | Not mentioned | Nasiri et al., 2021 [221] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo-Lozada, G.M.; Villarruel-López, A.; Nuño, K.; García-García, A.; Sánchez-Nuño, Y.A.; Ramos-García, C.O. Clinically Effective Molecules of Natural Origin for Obesity Prevention or Treatment. Int. J. Mol. Sci. 2024, 25, 2671. https://doi.org/10.3390/ijms25052671
Hidalgo-Lozada GM, Villarruel-López A, Nuño K, García-García A, Sánchez-Nuño YA, Ramos-García CO. Clinically Effective Molecules of Natural Origin for Obesity Prevention or Treatment. International Journal of Molecular Sciences. 2024; 25(5):2671. https://doi.org/10.3390/ijms25052671
Chicago/Turabian StyleHidalgo-Lozada, Gladys Maribel, Angelica Villarruel-López, Karla Nuño, Abel García-García, Yaír Adonaí Sánchez-Nuño, and César Octavio Ramos-García. 2024. "Clinically Effective Molecules of Natural Origin for Obesity Prevention or Treatment" International Journal of Molecular Sciences 25, no. 5: 2671. https://doi.org/10.3390/ijms25052671
APA StyleHidalgo-Lozada, G. M., Villarruel-López, A., Nuño, K., García-García, A., Sánchez-Nuño, Y. A., & Ramos-García, C. O. (2024). Clinically Effective Molecules of Natural Origin for Obesity Prevention or Treatment. International Journal of Molecular Sciences, 25(5), 2671. https://doi.org/10.3390/ijms25052671