Raman Spectroscopy as a Research and Diagnostic Tool in Clinical Hematology and Hematooncology
Abstract
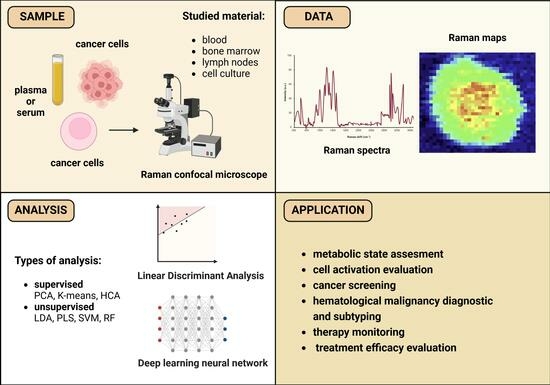
:1. Introduction
2. Raman Spectroscopy as a Research Tool in Medicine
2.1. General Principles of Raman Spectroscopy
2.2. Visualization and Analysis of Raman Spectra
2.3. Applicability of Raman Spectroscopy to Medical Applications
3. Applications of Raman Spectroscopy in Medical Research and Clinical Studies
3.1. Raman Spectroscopy in Healthy Hematopoietic Cells
3.1.1. Hematopoietic Stem Cells
3.1.2. Lymphocytes
3.1.3. Monocytes and Macrophages
3.1.4. Future Perspectives
3.2. Raman Spectroscopy in Tumor Studies
3.2.1. Cancer Screening
3.2.2. Hematopoietic Malignancy Diagnostics and Subtyping
3.2.3. Therapy Monitoring and Treatment Efficacy Evaluation
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, J.; Xiao, W.; Abdel-Wahab, O. Diagnosis and classification of hematologic malignancies on the basis of genetics. Blood 2017, 130, 410–423. [Google Scholar] [CrossRef]
- Woo, J.; Baumann, A.; Arguello, V. Recent advancements of flow cytometry: New applications in hematology and oncology. Expert Rev. Mol. Diagn. 2014, 14, 67–81. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed]
- Bacher, U.; Shumilov, E.; Flach, J.; Porret, N.; Joncourt, R.; Wiedemann, G.; Fiedler, M.; Novak, U.; Amstutz, U.; Pabst, T. Challenges in the introduction of next-generation sequencing (NGS) for diagnostics of myeloid malignancies into clinical routine use. Blood Cancer J. 2018, 8, 113. [Google Scholar] [CrossRef]
- Kong, K.; Kendall, C.; Stone, N.; Notingher, I. Raman spectroscopy for medical diagnostics—From in-vitro biofluid assays to in-vivo cancer detection. Adv. Drug Deliv. Rev. 2015, 89, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-L.; Wang, J.-H.; Zhao, A.-H.; Xu, X.; Wang, Y.-H.; Chen, T.-L.; Li, J.-M.; Mi, J.-Q.; Zhu, Y.-M.; Liu, Y.-F.; et al. A distinct glucose metabolism signature of acute myeloid leukemia with prognostic value. Blood 2014, 124, 1645–1654. [Google Scholar] [CrossRef]
- Dobson, S.M.; García-Prat, L.; Vanner, R.J.; Wintersinger, J.; Waanders, E.; Gu, Z.; McLeod, J.; Gan, O.I.; Grandal, I.; Payne-Turner, D.; et al. Relapse-Fated Latent Diagnosis Subclones in Acute B Lineage Leukemia Are Drug Tolerant and Possess Distinct Metabolic Programs. Cancer Discov. 2020, 10, 568–587. [Google Scholar] [CrossRef]
- Baranska, M. (Ed.) Optical Spectroscopy and Computational Methods in Biology and Medicine; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Raman, C.V.; Krishnan, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Auner, G.W.; Koya, S.K.; Huang, C.; Broadbent, B.; Trexler, M.; Auner, Z.; Elias, A.; Mehne, K.C.; Brusatori, M.A. Applications of Raman spectroscopy in cancer diagnosis. Cancer Metastasis Rev. 2018, 37, 691–717. [Google Scholar] [CrossRef]
- Palonpon, A.F.; Sodeoka, M.; Fujita, K. Molecular imaging of live cells by Raman microscopy. Curr. Opin. Chem. Biol. 2013, 17, 708–715. [Google Scholar] [CrossRef]
- Naumann, M.; Arend, N.; Guliev, R.R.; Kretzer, C.; Rubio, I.; Werz, O.; Neugebauer, U. Label-Free Characterization of Macrophage Polarization Using Raman Spectroscopy. Int. J. Mol. Sci. 2023, 24, 824. [Google Scholar] [CrossRef]
- Lee, L.C.; Liong, C.-Y.; Jemain, A.A. Partial least squares-discriminant analysis (PLS-DA) for classification of high-dimensional (HD) data: A review of contemporary practice strategies and knowledge gaps. Analyst 2018, 143, 3526–3539. [Google Scholar] [CrossRef]
- Taleb, A.; Diamond, J.; McGarvey, J.J.; Beattie, J.R.; Toland, C.; Hamilton, P.W. Raman Microscopy for the Chemometric Analysis of Tumor Cells. J. Phys. Chem. B 2006, 110, 19625–19631. [Google Scholar] [CrossRef]
- Rohman, A.; Windarsih, A.; Lukitaningsih, E.; Rafi, M.; Betania, K.; Fadzillah, N.A. The use of FTIR and Raman spectroscopy in combination with chemometrics for analysis of biomolecules in biomedical fluids: A review. Biomed. Spectrosc. Imaging 2019, 8, 55–71. [Google Scholar] [CrossRef]
- Qi, Y.; Hu, D.; Jiang, Y.; Wu, Z.; Zheng, M.; Chen, E.X.; Liang, Y.; Sadi, M.A.; Zhang, K.; Chen, Y.P. Recent Progresses in Machine Learning Assisted Raman Spectroscopy. Adv. Opt. Mater. 2023, 11, 2203104. [Google Scholar] [CrossRef]
- Seifert, S. Application of random forest based approaches to surface-enhanced Raman scattering data. Sci. Rep. 2020, 10, 5436. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Zhang, W.; Chen, C.; Zhang, H.; Zhou, A.; Huang, Y. Tracking the Differentiation Status of Human Neural Stem Cells through Label-Free Raman Spectroscopy and Machine Learning-Based Analysis. Anal. Chem. 2021, 93, 10453–10461. [Google Scholar] [CrossRef] [PubMed]
- Denbigh, J.L.; Perez-Guaita, D.; Vernooij, R.R.; Tobin, M.J.; Bambery, K.R.; Xu, Y.; Southam, A.D.; Khanim, F.L.; Drayson, M.T.; Lockyer, N.P.; et al. Probing the action of a novel anti-leukaemic drug therapy at the single cell level using modern vibrational spectroscopy techniques. Sci. Rep. 2017, 7, 2649. [Google Scholar] [CrossRef] [PubMed]
- Baczewska, M.; Królikowska, M.; Mazur, M.; Laskowska, P.; Dziekan, Z.; Mrówka, P.; Krauze, W.; Kujawińska, M. (Eds.) Quantitative phase imaging supported by raman micro-spectroscopy for identifying and quantifying changes in myeloid cells treated with proteasome inhibitor. In Proceedings of the Quantitative Phase Imaging IX, San Francisco, CA, USA, 28–30 January 2023. [Google Scholar]
- Kast, R.; Auner, G.; Yurgelevic, S.; Broadbent, B.; Raghunathan, A.; Poisson, L.M.; Mikkelsen, T.; Rosenblum, M.L.; Kalkanis, S.N. Identification of regions of normal grey matter and white matter from pathologic glioblastoma and necrosis in frozen sections using Raman imaging. J. Neuro-Oncol. 2015, 125, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Morais, C.L.M.; Martin-Hirsch, P.L.; Martin, F.L. A three-dimensional principal component analysis approach for exploratory analysis of hyperspectral data: Identification of ovarian cancer samples based on Raman microspectroscopy imaging of blood plasma. Analyst 2019, 144, 2312–2319. [Google Scholar] [CrossRef]
- Blanco-Formoso, M.; Alvarez-Puebla, R.A. Cancer Diagnosis through SERS and other Related Techniques. Int. J. Mol. Sci. 2020, 21, 2253. [Google Scholar] [CrossRef]
- Grieve, S.; Puvvada, N.; Phinyomark, A.; Russell, K.; Murugesan, A.; Zed, E.; Hassan, A.; Legare, J.-F.; Kienesberger, P.C.; Pulinilkunnil, T.; et al. Nanoparticle surface-enhanced Raman spectroscopy as a noninvasive, label-free tool to monitor hematological malignancy. Nanomedicine 2021, 16, 2175–2188. [Google Scholar] [CrossRef]
- DOLPHIN-VIVO: Diagnosis of LymPHoma In Vivo (Ex Vivo Phase). Identifier NCT04162431. U.S. National Library of Medicine. Available online: https://clinicaltrials.gov/study/NCT04162431 (accessed on 15 January 2024).
- Mondol, A.S.; Töpfer, N.; Rüger, J.; Neugebauer, U.; Popp, J.; Schie, I.W. New perspectives for viability studies with high-content analysis Raman spectroscopy (HCA-RS). Sci. Rep. 2019, 9, 12653. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.R.; Hooper, D.C.; Zhang, L.; Wolverson, D.; Valev, V.K. Raman Techniques: Fundamentals and Frontiers. Nanoscale Res. Lett. 2019, 14, 231. [Google Scholar] [CrossRef] [PubMed]
- Sepp, K.; Lee, M.; Bluntzer, M.T.J.; Helgason, G.V.; Hulme, A.N.; Brunton, V.G. Utilizing Stimulated Raman Scattering Microscopy To Study Intracellular Distribution of Label-Free Ponatinib in Live Cells. J. Med. Chem. 2020, 63, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
- Praveen, B.B.; Mazilu, M.; Marchington, R.F.; Herrington, C.S.; Riches, A.; Dholakia, K. Optimisation of Wavelength Modulated Raman Spectroscopy: Towards High Throughput Cell Screening. PLoS ONE 2013, 8, e67211. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; McReynolds, N.; Campbell, E.C.; Mazilu, M.; Barbosa, J.; Dholakia, K.; Powis, S.J. The Use of Wavelength Modulated Raman Spectroscopy in Label-Free Identification of T Lymphocyte Subsets, Natural Killer Cells and Dendritic Cells. PLoS ONE 2015, 10, e0125158. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.J.; Wang, J.-C.E.; Quataert, S.A.; Berger, A.J. Integrated Raman and angular scattering microscopy reveals chemical and morphological differences between activated and nonactivated CD8+ T lymphocytes. J. Biomed. Opt. 2010, 15, 36021. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, T.; Chiu, L.-d.; Fujita, K.; Machiyama, H.; Yamaguchi, T.; Watanabe, T.M.; Fujita, H. Non-label immune cell state prediction using Raman spectroscopy. Sci. Rep. 2016, 6, 37562. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Que Nguyen, T.N.; Maguire, A.; Wynne, C.; Meade, A.D. Comparison of sample preparation methodologies towards optimisation of Raman spectroscopy for peripheral blood mononuclear cells. Anal. Methods 2021, 13, 1019–1032. [Google Scholar] [CrossRef]
- Rigaud, V.O.C.; Hoy, R.; Mohsin, S.; Khan, M. Stem Cell Metabolism: Powering Cell-Based Therapeutics. Cells 2020, 9, 2490. [Google Scholar] [CrossRef]
- Harkness, L.; Novikov, S.M.; Beermann, J.; Bozhevolnyi, S.I.; Kassem, M. Identification of abnormal stem cells using Raman spectroscopy. Stem Cells Dev. 2012, 21, 2152–2159. [Google Scholar] [CrossRef]
- Chan, J.W.; Lieu, D.K.; Huser, T.; Li, R.A. Label-Free Separation of Human Embryonic Stem Cells and Their Cardiac Derivatives Using Raman Spectroscopy. Anal. Chem. 2009, 81, 1324–1331. [Google Scholar] [CrossRef]
- Alraies, A.; Canetta, E.; Waddington, R.J.; Moseley, R.; Sloan, A.J. Discrimination of Dental Pulp Stem Cell Regenerative Heterogeneity by Single-Cell Raman Spectroscopy. Tissue Eng. Part C Methods 2019, 25, 489–499. [Google Scholar] [CrossRef]
- Alattar, N.; Daud, H.; Al-Majmaie, R.; Zeulla, D.; Al-Rubeai, M.; Rice, J.H. Surface-enhanced Raman scattering for rapid hematopoietic stem cell differentiation analysis. Appl. Opt. 2018, 57, E184–E189. [Google Scholar] [CrossRef]
- Ilin, Y.; Choi, J.S.; Harley, B.A.C.; Kraft, M.L. Identifying States along the Hematopoietic Stem Cell Differentiation Hierarchy with Single Cell Specificity via Raman Spectroscopy. Anal. Chem. 2015, 87, 11317–11324. [Google Scholar] [CrossRef]
- Pastrana-Otero, I.; Majumdar, S.; Gilchrist, A.E.; Harley, B.A.C.; Kraft, M.L. Identification of the Differentiation Stages of Living Cells from the Six Most Immature Murine Hematopoietic Cell Populations by Multivariate Analysis of Single-Cell Raman Spectra. Anal. Chem. 2022, 94, 11999–12007. [Google Scholar] [CrossRef]
- Choi, J.S.; Ilin, Y.; Kraft, M.L.; Harley, B.A.C. Tracing Hematopoietic Progenitor Cell Neutrophilic Differentiation via Raman Spectroscopy. Bioconjugate Chem. 2018, 29, 3121–3128. [Google Scholar] [CrossRef]
- Nitta, N.; Iino, T.; Isozaki, A.; Yamagishi, M.; Kitahama, Y.; Sakuma, S.; Suzuki, Y.; Tezuka, H.; Oikawa, M.; Arai, F.; et al. Raman image-activated cell sorting. Nat. Commun. 2020, 11, 3452. [Google Scholar] [CrossRef]
- Lee, K.S.; Pereira, F.C.; Palatinszky, M.; Behrendt, L.; Alcolombri, U.; Berry, D.; Wagner, M.; Stocker, R. Optofluidic Raman-activated cell sorting for targeted genome retrieval or cultivation of microbial cells with specific functions. Nat. Protoc. 2021, 16, 634–676. [Google Scholar] [CrossRef]
- Fore, S.; Chan, J.; Taylor, D.; Huser, T. Raman spectroscopy of individual monocytes reveals that single-beam optical trapping of mononuclear cells occurs by their nucleus. J. Opt. 2011, 13, 44021. [Google Scholar] [CrossRef]
- Chaudhary, N.; Nguyen, T.N.Q.; Cullen, D.; Meade, A.D.; Wynne, C. Discrimination of immune cell activation using Raman micro-spectroscopy in an in-vitro & ex-vivo model. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119118. [Google Scholar] [CrossRef]
- Borek-Dorosz, A.; Nowakowska, A.M.; Leszczenko, P.; Adamczyk, A.; Pieczara, A.; Jakubowska, J.; Pastorczak, A.; Ostrowska, K.; Ząbczyńska, M.; Sowinski, K.; et al. Raman-based spectrophenotyping of the most important cells of the immune system. J. Adv. Res. 2022, 41, 191–203. [Google Scholar] [CrossRef]
- Managò, S.; Mirabelli, P.; Napolitano, M.; Zito, G.; de Luca, A.C. Raman detection and identification of normal and leukemic hematopoietic cells. J. Biophotonics 2018, 11, e201700265. [Google Scholar] [CrossRef]
- Li, W.; Wang, L.; Luo, C.; Zhu, Z.; Ji, J.; Pang, L.; Huang, Q. Characteristic of Five Subpopulation Leukocytes in Single-Cell Levels Based on Partial Principal Component Analysis Coupled with Raman Spectroscopy. Appl. Spectrosc. 2020, 74, 1463–1472. [Google Scholar] [CrossRef]
- Mannie, M.D.; McConnell, T.J.; Xie, C.; Li, Y.-q. Activation-dependent phases of T cells distinguished by use of optical tweezers and near infrared Raman spectroscopy. J. Immunol. Methods 2005, 297, 53–60. [Google Scholar] [CrossRef]
- Brown, K.L.; Palyvoda, O.Y.; Thakur, J.S.; Nehlsen-Cannarella, S.L.; Fagoaga, O.R.; Gruber, S.A.; Auner, G.W. Raman spectroscopic differentiation of activated versus non-activated T lymphocytes: An in vitro study of an acute allograft rejection model. J. Immunol. Methods 2009, 340, 48–54. [Google Scholar] [CrossRef]
- Brown, K.L.; Palyvoda, O.Y.; Auner, G.W.; Gruber, S.A. Raman spectroscopic modeling of early versus late T-lymphocyte activation via differential spectral detection of receptor expression. J. Immunol. Methods 2014, 415, 31–35. [Google Scholar] [CrossRef]
- Pavillon, N.; Smith, N.I. Non-invasive monitoring of T cell differentiation through Raman spectroscopy. Sci. Rep. 2023, 13, 3129. [Google Scholar] [CrossRef]
- Morrish, R.; Yim, K.H.W.; Pagliara, S.; Palombo, F.; Chahwan, R.; Stone, N. Single Cell Label-Free Probing of Chromatin Dynamics During B Lymphocyte Maturation. Front. Cell Dev. Biol. 2021, 9, 646616. [Google Scholar] [CrossRef]
- Robert, C.; Tsiampali, J.; Fraser-Miller, S.J.; Neumann, S.; Maciaczyk, D.; Young, S.L.; Maciaczyk, J.; Gordon, K.C. Molecular monitoring of glioblastoma’s immunogenicity using a combination of Raman spectroscopy and chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 252, 119534. [Google Scholar] [CrossRef] [PubMed]
- Ramoji, A.; Thomas-Rüddel, D.; Ryabchykov, O.; Bauer, M.; Arend, N.; Giamarellos-Bourboulis, E.J.; Eugen-Olsen, J.; Kiehntopf, M.; Bocklitz, T.; Popp, J.; et al. Leukocyte Activation Profile Assessed by Raman Spectroscopy Helps Diagnosing Infection and Sepsis. Crit. Care Explor. 2021, 3, e0394. [Google Scholar] [CrossRef] [PubMed]
- Töpfer, N.; Müller, M.M.; Dahms, M.; Ramoji, A.; Popp, J.; Slevogt, H.; Neugebauer, U. Raman spectroscopy reveals LPS-induced changes of biomolecular composition in monocytic THP-1 cells in a label-free manner. Integr. Biol. 2019, 11, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Ribeiro, A.R.B.; Silva, E.C.O.; Araújo, P.M.C.; Souza, S.T.; Fonseca, E.J.d.S.; Barreto, E. Application of Raman spectroscopy for characterization of the functional polarization of macrophages into M1 and M2 cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 265, 120328. [Google Scholar] [CrossRef]
- Feuerer, N.; Marzi, J.; Brauchle, E.M.; Carvajal Berrio, D.A.; Billing, F.; Weiss, M.; Jakobi, M.; Schneiderhan-Marra, N.; Shipp, C.; Schenke-Layland, K. Lipidome profiling with Raman microspectroscopy identifies macrophage response to surface topographies of implant materials. Proc. Natl. Acad. Sci. USA 2021, 118, e2113694118. [Google Scholar] [CrossRef]
- Batista-Gonzalez, A.; Vidal, R.; Criollo, A.; Carreño, L.J. New Insights on the Role of Lipid Metabolism in the Metabolic Reprogramming of Macrophages. Front. Immunol. 2019, 10, 2993. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, X.; Jansen, J.A.; Yang, F.; van den Beucken, J.J.J.P. Titanium surfaces characteristics modulate macrophage polarization. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 95, 143–151. [Google Scholar] [CrossRef]
- Woods, F.E.R.; Chandler, S.; Sikora, N.; Harford, R.; Souriti, A.; Gray, H.; Wilkes, H.; Lloyd-Bennett, C.; Harris, D.A.; Dunstan, P.R. An observational cohort study to evaluate the use of serum Raman spectroscopy in a rapid diagnosis center setting. Clin. Spectrosc. 2022, 4, 100020. [Google Scholar] [CrossRef]
- Tallerico, R.; Todaro, M.; Di Franco, S.; Maccalli, C.; Garofalo, C.; Sottile, R.; Palmieri, C.; Tirinato, L.; Pangigadde, P.N.; La Rocca, R.; et al. Human NK cells selective targeting of colon cancer-initiating cells: A role for natural cytotoxicity receptors and MHC class I molecules. J. Immunol. 2013, 190, 2381–2390. [Google Scholar] [CrossRef]
- Ishwar, D.; Haldavnekar, R.; Venkatakrishnan, K.; Tan, B. Minimally invasive detection of cancer using metabolic changes in tumor-associated natural killer cells with Oncoimmune probes. Nat. Commun. 2022, 13, 4527. [Google Scholar] [CrossRef]
- Katsara, K.; Psatha, K.; Kenanakis, G.; Aivaliotis, M.; Papadakis, V.M. Subtyping on Live Lymphoma Cell Lines by Raman Spectroscopy. Materials 2022, 15, 546. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, M.; Köse-Vogel, N.; Kiselev, R.; Kirchberger-Tolstik, T.; Schie, I.; Krafft, C.; Popp, J. Quantitation of acute monocytic leukemia cells spiked in control monocytes using surface-enhanced Raman spectroscopy. Anal. Methods 2018, 10, 2785–2791. [Google Scholar] [CrossRef]
- Yu, Y.; Lin, J.; Lin, D.; Feng, S.; Chen, W.; Huang, Z.; Huang, H.; Chen, R. Leukemia cells detection based on electroporation assisted surface-enhanced Raman scattering. Biomed. Opt. Express 2017, 8, 4108–4121. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.M.; de Siqueira E Oliveira, F.S.A.; de Brito, P.L.; Silveira, L. Spectral model for diagnosis of acute leukemias in whole blood and plasma through Raman spectroscopy. J. Biomed. Opt. 2018, 23, 107002. [Google Scholar] [CrossRef]
- Bai, Y.; Yu, Z.; Yi, S.; Yan, Y.; Huang, Z.; Qiu, L. Raman spectroscopy-based biomarker screening by studying the fingerprint characteristics of chronic lymphocytic leukemia and diffuse large B-cell lymphoma. J. Pharm. Biomed. Anal. 2020, 190, 113514. [Google Scholar] [CrossRef] [PubMed]
- Agsalda-Garcia, M.; Shieh, T.; Souza, R.; Kamada, N.; Loi, N.; Oda, R.; Acosta-Maeda, T.; Choi, S.Y.; Lim, E.; Misra, A.; et al. Raman-Enhanced Spectroscopy (RESpect) Probe for Childhood Non-Hodgkin Lymphoma. SciMed. J. 2020, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shiramizu, B.; Oda, R.; Kamada, N.; Garcia, M.A.; Shieh, T.; Maeda, T.A.; Choi, S.Y.; Lim, E.; Misra, A. Unique Raman Spectroscopic Fingerprints of B-Cell Non-Hodgkin Lymphoma: Implications for Diagnosis, Prognosis and New Therapies. J. Biol. Med. Sci. 2018, 2, 105. [Google Scholar]
- Rau, J.V.; Marini, F.; Fosca, M.; Cippitelli, C.; Rocchia, M.; Di Napoli, A. Raman spectroscopy discriminates malignant follicular lymphoma from benign follicular hyperplasia and from tumour metastasis. Talanta 2019, 194, 763–770. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.d.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Yang, H.; Xie, J.; Liu, A. Diagnosis and staging of diffuse large B-cell lymphoma using label-free surface-enhanced Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 267, 120571. [Google Scholar] [CrossRef]
- Dawiec, P.; Leszczenko, P.; Nowakowska, A.M.; Laskowska, P.; Szydłowski, M.; Juszczyński, P.; Baranska, M.; Mrówka, P.; Majzner, K. Automatic subtyping of Diffuse Large B-cell Lymphomas (DLBCL): Raman-based genetic and metabolic classification. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 309, 123795. [Google Scholar] [CrossRef]
- Managò, S.; Valente, C.; Mirabelli, P.; Circolo, D.; Basile, F.; Corda, D.; de Luca, A.C. A reliable Raman-spectroscopy-based approach for diagnosis, classification and follow-up of B-cell acute lymphoblastic leukemia. Sci. Rep. 2016, 6, 24821. [Google Scholar] [CrossRef]
- Vanna, R.; Ronchi, P.; Lenferink, A.; Tresoldi, C.; Morasso, C.; Mehn, D.; Bedoni, M.; Picciolini, S.; Terstappen, L.; Ciceri, F.; et al. Label-free imaging and identification of typical cells of acute myeloid leukaemia and myelodysplastic syndrome by Raman microspectroscopy. Analyst 2014, 140, 1054. [Google Scholar] [CrossRef]
- Liang, H.; Kong, X.; Wang, H.; Ren, Y.; Liu, E.; Sun, F.; Qi, J.; Zhang, Q.; Zhou, Y. Elucidating the Heterogeneity of Serum Metabolism in Patients with Myelodysplastic Syndrome and Acute Myeloid Leukemia by Raman Spectroscopy. ACS Omega 2022, 7, 47056–47069. [Google Scholar] [CrossRef]
- Garcia-Manero, G. Myelodysplastic syndromes: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2023, 98, 1307–1325. [Google Scholar] [CrossRef]
- Russo, M.; Tirinato, L.; Scionti, F.; Coluccio, M.L.; Perozziello, G.; Riillo, C.; Mollace, V.; Gratteri, S.; Malara, N.; Di Martino, M.T.; et al. Raman Spectroscopic Stratification of Multiple Myeloma Patients Based on Exosome Profiling. ACS Omega 2020, 5, 30436–30443. [Google Scholar] [CrossRef]
- Riva, G.; Nasillo, V.; Ottomano, A.M.; Bergonzini, G.; Paolini, A.; Forghieri, F.; Lusenti, B.; Barozzi, P.; Lagreca, I.; Fiorcari, S.; et al. Multiparametric Flow Cytometry for MRD Monitoring in Hematologic Malignancies: Clinical Applications and New Challenges. Cancers 2021, 13, 4582. [Google Scholar] [CrossRef]
- Franco, D.; Trusso, S.; Fazio, E.; Allegra, A.; Musolino, C.; Speciale, A.; Cimino, F.; Saija, A.; Neri, F.; Nicolò, M.S.; et al. Raman spectroscopy differentiates between sensitive and resistant multiple myeloma cell lines. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 187, 15–22. [Google Scholar] [CrossRef]
- Kang, J.W.; Singh, S.P.; Nguyen, F.T.; Lue, N.; Sung, Y.; So, P.T.C.; Dasari, R.R. Investigating Effects of Proteasome Inhibitor on Multiple Myeloma Cells Using Confocal Raman Microscopy. Sensors 2016, 16, 2133. [Google Scholar] [CrossRef]
- Murray, J.A.; Khanim, F.L.; Hayden, R.E.; Craddock, C.F.; Holyoake, T.L.; Jackson, N.; Lumley, M.; Bunce, C.M.; Drayson, M.T. Combined bezafibrate and medroxyprogesterone acetate have efficacy without haematological toxicity in elderly and relapsed acute myeloid leukaemia (AML). Br. J. Haematol. 2010, 149, 65–69. [Google Scholar] [CrossRef]
- Liu, X.; Kung, A.; Malinoski, B.; Prakash, G.K.S.; Zhang, C. Development of Alkyne-Containing Pyrazolopyrimidines To Overcome Drug Resistance of Bcr-Abl Kinase. J. Med. Chem. 2015, 58, 9228–9237. [Google Scholar] [CrossRef]
- Lin, T.L.; Vala, M.S.; Barber, J.P.; Karp, J.E.; Smith, B.D.; Matsui, W.; Jones, R.J. Induction of acute lymphocytic leukemia differentiation by maintenance therapy. Leukemia 2007, 21, 1915–1920. [Google Scholar] [CrossRef]
- Xiong, M.; Ye, J. Reproducibility in surface-enhanced Raman spectroscopy. J. Shanghai Jiaotong Univ. 2014, 19, 681–690. [Google Scholar] [CrossRef]
- Tsikritsis, D.; Legge, E.; Belsey, N. Practical considerations for quantitative and reproducible measurements with stimulated Raman scattering microscopy. Analyst 2022, 147, 4642–4656. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Chi, M.; Li, M.; Gao, S.; Su, L.; Han, W. Raman identification of single cell component and FMS-like tyrosine kinase 3-internal tandem duplications subtype for clinical acute myeloid leukemia. J. Raman Spectrosc. 2023, 54, 596–607. [Google Scholar] [CrossRef]
- Laskowska, P.; Orzechowska, S.; Borek-Dorosz, A.; Nowakowska, A.; Adamczyk, A.; Leszczenko, P.; Szydłowski, M.; Szlachetka, A.; Zasowska, M.; Juszczyński, P.; et al. 3137—Acute myloid leukemia key mutations in the lens of raman microscopy. Exp. Hematol. 2023, 124, S118. [Google Scholar] [CrossRef]
- Morla-Folch, J.; Gisbert-Quilis, P.; Masetti, M.; Garcia-Rico, E.; Alvarez-Puebla, R.A.; Guerrini, L. Conformational SERS Classification of K-Ras Point Mutations for Cancer Diagnostics. Angew. Chem. Int. Ed. 2017, 56, 2381–2385. [Google Scholar] [CrossRef]
- Huang, S.-q.; Hu, J.; Zhu, G.; Zhang, C.-y. Sensitive detection of point mutation using exponential strand displacement amplification-based surface enhanced Raman spectroscopy. Biosens. Bioelectron. 2015, 65, 191–197. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Krajczewski, J.; Kowalik, A.; Weyher, J.L.; Dzięcielewski, I.; Chłopek, M.; Góźdź, S.; Nowicka, A.M.; Kudelski, A. New strategy for the gene mutation identification using surface enhanced Raman spectroscopy (SERS). Biosens. Bioelectron. 2019, 132, 326–332. [Google Scholar] [CrossRef]
- Sciortino, T.; Secoli, R.; d’Amico, E.; Moccia, S.; Conti Nibali, M.; Gay, L.; Rossi, M.; Pecco, N.; Castellano, A.; de Momi, E.; et al. Raman Spectroscopy and Machine Learning for IDH Genotyping of Unprocessed Glioma Biopsies. Cancers 2021, 13, 4196. [Google Scholar] [CrossRef] [PubMed]
- Snyder, V.; Reed-Newman, T.C.; Arnold, L.; Thomas, S.M.; Anant, S. Cancer Stem Cell Metabolism and Potential Therapeutic Targets. Front. Oncol. 2018, 8, 203. [Google Scholar] [CrossRef] [PubMed]


| Technology/Modification | Raman Limitation Addressed | Principle | Use In Research/Diagnostics | Reference |
|---|---|---|---|---|
| Raman hyperspectral imaging (his) | In vivo tissue visualization with high resolution | Combines spectral information with spatial information to generate image showing distribution of biochemical components within the sample | Distinguishing of grey matter, white matter, GBM and necrosis within the sample | [21] |
| Distinguishing between ovarian cancer and healthy plasma samples | [22] | |||
| Surfaced-enhanced Raman spectroscopy (SERS) | Low sensitivity | By addition of a metal (Au or Ag), enhances the Raman scattering signal of molecules close to the surface and eliminates fluorescent background | Establishing a predictive model to evaluate changes in the disease progression over time within patients with MM or lymphoma | [23,24] |
| Fourier- transformation infrared (FTIR) Raman spectroscopy | Measures the absorption, reflection, or transmission of electromagnetic radiation in mid-infrared complimenting chemical data obtained from RS | Vibrational spectroscopy signal characterization in head and neck lymph nodes (via Raman and FTIR mapping measurements of tissue sections) | [15,25] | |
| High-content analysis Raman spectroscopy (HcA-RS) | Lack of automatization | Enables the sampling of a large number of cells under various physiological conditions without requiring user interaction | Spectral measurement of large number of samples—>25,000 spectra obtained for set of analysis | [26] |
| Stimulated Raman spectroscopy (SRS) | Slow acquisition | Provides much stronger signal which speeds the acquisition and eliminates a non-resonant background of spontaneous RS. With a linear relationship between signal intensity and chemical concentration, enables quantitative imaging | Real-time measurements of ponatinib distribution in live CML cells with high sensitivity and resolution | [27,28] |
| Wavelength-modulation Raman spectroscopy (WMRS) | suppresses the Raman background and speeds the acquisition time | Identification of T cells, NK cells and dendritic cells | [29,30] | |
| Integrated Raman and angular-scattering microscopy (IRAM) | Lack of morphological information | Simultaneous measurements of elastic and inelastic scattering | Chemical and morphological distinction between activated and non-activated CD8+ T lymphocytes | [31] |
| Cell Population | Application | Raman Technique | Reference |
|---|---|---|---|
| lymphocytes | activation status assessment | RS, SRS, FTIR Raman, IRAM | [31,33,49,50,51] |
| differentiation and maturation | RS | [52,53] | |
| leukocytes | distinction between healthy and leukocytes with inflammation. | RS | [55] |
| monocytes and macrophages | activation status assessment | RS | [33,56,59] |
| polarization | RS | [12,58,59] | |
| HSC and progenitor cells | classification and fate prediction | RS | [39,40,41] |
| Studied Sample | Application | Raman Technique | Reference |
|---|---|---|---|
| Serum | Solid tumor detection | RS | [62] |
| DLBCL diagnosis and staging | SERS | [74] | |
| Blood plasma | CLL screening | SERS | [69] |
| DLBCL screening | SERS | [69] | |
| AML screening | RS | [68] | |
| MM screening | RS, SERS | [76,81] | |
| Tumor-associated NK cells | Solid tumor detection | SERS | [64] |
| ALL cells | Distinction from healthy cells | RS | [47] |
| Subtyping | RS | [76] | |
| Treatment monitoring | RS | [76] | |
| NHL cells | Subtyping | RS | [65] |
| Screening (diagnosis) | RS, RESpect (SERS), FTIR Raman | [25,70,71] | |
| AML cells | Distinction from healthy cells | SERS | [66,67] |
| Subtyping (based on FAB classification) | RS | [77] | |
| Differentiation from MDS | RS | [77,78] | |
| Treatment monitoring | HcA-RS, FTIR Raman | [19,26] | |
| DLBCL | Subtype classification | RS, SERS | [75,80] |
| Classification and staging | RS | [72] | |
| MM | Drug resistance/ sensitivity studies | RS | [82] |
| Treatment monitoring | RS, NIRS | [20,83] | |
| Diagnosis and MRD detection | RS, SERS | [76,81] | |
| CML | Treatment monitoring | SRS | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laskowska, P.; Mrowka, P.; Glodkowska-Mrowka, E. Raman Spectroscopy as a Research and Diagnostic Tool in Clinical Hematology and Hematooncology. Int. J. Mol. Sci. 2024, 25, 3376. https://doi.org/10.3390/ijms25063376
Laskowska P, Mrowka P, Glodkowska-Mrowka E. Raman Spectroscopy as a Research and Diagnostic Tool in Clinical Hematology and Hematooncology. International Journal of Molecular Sciences. 2024; 25(6):3376. https://doi.org/10.3390/ijms25063376
Chicago/Turabian StyleLaskowska, Paulina, Piotr Mrowka, and Eliza Glodkowska-Mrowka. 2024. "Raman Spectroscopy as a Research and Diagnostic Tool in Clinical Hematology and Hematooncology" International Journal of Molecular Sciences 25, no. 6: 3376. https://doi.org/10.3390/ijms25063376
APA StyleLaskowska, P., Mrowka, P., & Glodkowska-Mrowka, E. (2024). Raman Spectroscopy as a Research and Diagnostic Tool in Clinical Hematology and Hematooncology. International Journal of Molecular Sciences, 25(6), 3376. https://doi.org/10.3390/ijms25063376