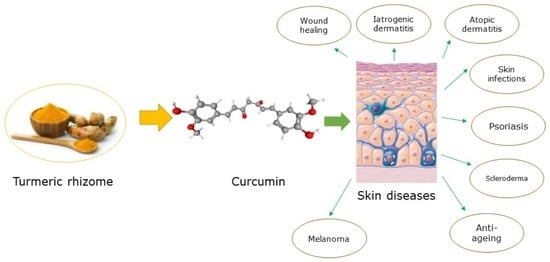
Potential of Curcumin in the Management of Skin Diseases
Abstract
:1. Introduction
2. Curcumin and Skin Diseases
2.1. Psoriasis
2.2. Scleroderma
2.3. Atopic Dermatitis
2.4. Iatrogenic Dermatitis
2.5. Wound Healing
2.6. Skin Infections
2.6.1. Antibacterial Activity
2.6.2. Antifungal Activity
2.6.3. Antiparasites Activity
2.6.4. Antiviral Activity
2.7. Anti-Aging/Angiogenesis
3. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamer-Zarawska, E. Fitoterapia i Leki Roślinne; PZWL Wydawnictwo Lekarskie: Warszawa, Poland, 2013; ISBN 978-83-20-04650-2. [Google Scholar]
- Frei, G.; Haimhoffer, Á.; Csapó, E.; Bodnár, K.; Vasvári, G.; Nemes, D.; Lekli, I.; Gyöngyösi, A.; Bácskay, I.; Fehér, P.; et al. In Vitro and In Vivo Efficacy of Topical Dosage Forms Containing Self-Nanoemulsifying Drug Delivery System Loaded with Curcumin. Pharmaceutics 2023, 15, 2054. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Curcumin: A Review of Clinical Trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Zucca, P.; Sharifi-Rad, M.; Pezzani, R.; Rajabi, S.; Setzer, W.N.; Varoni, E.M.; Iriti, M.; Kobarfard, F.; Sharifi-Rad, J. Phytotherapeutics in Cancer Invasion and Metastasis. Phytother. Res. 2018, 32, 1425–1449. [Google Scholar] [CrossRef] [PubMed]
- Purpura, M.; Lowery, R.P.; Wilson, J.M.; Mannan, H.; Münch, G.; Razmovski-Naumovski, V. Analysis of Different Innovative Formulations of Curcumin for Improved Relative Oral Bioavailability in Human Subjects. Eur. J. Nutr. 2018, 57, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Terlikowska, K.; Witkowska, A.; Terlikowski, S. Kurkumina w chemoprewencji raka piersi. Postep. Hig. Med. Dosw. 2014, 68, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, Q.; Wu, Z.; Xu, Y.; Jiang, R. Curcumin for Treating Breast Cancer: A Review of Molecular Mechanisms, Combinations with Anticancer Drugs, and Nanosystems. Pharmaceutics 2024, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Sharma, A. Turmeric (Curcuma longa): miRNAs and Their Regulating Targets Are Involved in Development and Secondary Metabolite Pathways. C. R. Biol. 2017, 340, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Aggarwal, R.; Prakash, U.; Sahoo, P.K. Emerging Therapeutic Potential of Curcumin in the Management of Dermatological Diseases: An Extensive Review of Drug and Pharmacological Activities. Future J. Pharm. Sci. 2023, 9, 42. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Gancarz, M.; Kondracka, A.; Rusinek, R.; Oniszczuk, A. Curcumin and Weight Loss: Does It Work? Int. J. Mol. Sci. 2022, 23, 639. [Google Scholar] [CrossRef]
- Toden, S.; Goel, A. The Holy Grail of Curcumin and Its Efficacy in Various Diseases: Is Bioavailability Truly a Big Concern? J. Restor. Med. 2017, 6, 27–36. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2012, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Rachmawati, H.; Edityaningrum, C.A.; Mauludin, R. Molecular Inclusion Complex of Curcumin-β-Cyclodextrin Nanoparticle to Enhance Curcumin Skin Permeability from Hydrophilic Matrix Gel. AAPS PharmSciTech 2013, 14, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, C.; Das, M.; Sahoo, S.K. Sustained Wound Healing Activity of Curcumin Loaded Oleic Acid Based Polymeric Bandage in a Rat Model. Mol. Pharm. 2012, 9, 2801–2811. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Nan, K.; Li, L.; Zhang, Z.; Chen, H. In Vivo Evaluation of Curcumin Nanoformulation Loaded Methoxy Poly(Ethylene Glycol)-Graft-Chitosan Composite Film for Wound Healing Application. Carbohydr. Polym. 2012, 88, 84–90. [Google Scholar] [CrossRef]
- El-Refaie, W.M.; Elnaggar, Y.S.R.; El-Massik, M.A.; Abdallah, O.Y. Novel Curcumin-Loaded Gel-Core Hyaluosomes with Promising Burn-Wound Healing Potential: Development, In-Vitro Appraisal and In-Vivo Studies. Int. J. Pharm. 2015, 486, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Merrell, J.G.; McLaughlin, S.W.; Tie, L.; Laurencin, C.T.; Chen, A.F.; Nair, L.S. Curcumin Loaded Poly(ε-Caprolactone) Nanofibers: Diabetic Wound Dressing with Antioxidant and Anti-Inflammatory Properties. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Madan, S.; Nehate, C.; Barman, T.K.; Rathore, A.S.; Koul, V. Design, Preparation, and Evaluation of Liposomal Gel Formulations for Treatment of Acne: In Vitro and in Vivo Studies. Drug Dev. Ind. Pharm. 2019, 45, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.-H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Damiani, G.; Bragazzi, N.L.; Karimkhani Aksut, C.; Wu, D.; Alicandro, G.; McGonagle, D.; Guo, C.; Dellavalle, R.; Grada, A.; Wong, P.; et al. The Global, Regional, and National Burden of Psoriasis: Results and Insights From the Global Burden of Disease 2019 Study. Front. Med. 2021, 8, 743180. [Google Scholar] [CrossRef]
- Kim, J.; Krueger, J.G. Highly Effective New Treatments for Psoriasis Target the IL-23/Type 17 T Cell Autoimmune Axis. Annu. Rev. Med. 2017, 68, 255–269. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Suárez-Fariñas, M.; Fuentes-Duculan, J.; Cueto, I.; Li, K.; Tian, S.; Brodmerkel, C.; Krueger, J.g. Increased Expression of Interleukin-17 Pathway Genes in Nonlesional Skin of Moderate-to-Severe Psoriasis Vulgaris. Br. J. Dermatol. 2016, 174, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Tomar, Y.; Gorantla, S.; Singhvi, G. Insight into the Pivotal Role of Signaling Pathways in Psoriasis Pathogenesis, Potential Therapeutic Molecules and Drug Delivery Approaches. Drug Discov. Today 2023, 28, 103465. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential Therapeutic Effects of Curcumin, the Anti-Inflammatory Agent, Against Neurodegenerative, Cardiovascular, Pulmonary, Metabolic, Autoimmune and Neoplastic Diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Heng, M.C.; Song, M.K.; Harker, J.; Heng, M.K. Drug-Induced Suppression of Phosphorylase Kinase Activity Correlates with Resolution of Psoriasis as Assessed by Clinical, Histological and Immunohistochemical Parameters. Br. J. Dermatol. 2000, 143, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Bahraini, P.; Rajabi, M.; Mansouri, P.; Sarafian, G.; Chalangari, R.; Azizian, Z. Turmeric Tonic as a Treatment in Scalp Psoriasis: A Randomized Placebo-Control Clinical Trial. J. Cosmet. Dermatol. 2018, 17, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Sarafian, G.; Afshar, M.; Mansouri, P.; Asgarpanah, J.; Raoufinejad, K.; Rajabi, M. Topical Turmeric Microemulgel in the Management of Plaque Psoriasis; A Clinical Evaluation. Iran. J. Pharm. Res. 2015, 14, 865–876. [Google Scholar] [PubMed]
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, S.; Webb, L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by Curcumin as Revealed by Molecular Interaction Studies. Nat. Prod. Rep. 2011, 28, 1937–1955. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhao, Y.; Hu, J. Curcumin Inhibits Imiquimod-Induced Psoriasis-Like Inflammation by Inhibiting IL-1β and IL-6 Production in Mice. PLoS ONE 2013, 8, e67078. [Google Scholar] [CrossRef]
- Kang, D.; Li, B.; Luo, L.; Jiang, W.; Lu, Q.; Rong, M.; Lai, R. Curcumin Shows Excellent Therapeutic Effect on Psoriasis in Mouse Model. Biochimie 2016, 123, 73–80. [Google Scholar] [CrossRef]
- Farag, A.G.A.; Shoaib, M.A.; Samaka, R.M.; Abdou, A.G.; Mandour, M.M.; Ibrahim, R.A.L. Progranulin and Beta-Catenin in Psoriasis: An Immunohistochemical Study. J. Cosmet. Dermatol. 2019, 18, 2019–2026. [Google Scholar] [CrossRef]
- Huang, K.; Chen, A.; Zhang, X.; Song, Z.; Xu, H.; Cao, J.; Yin, Y. Progranulin Is Preferentially Expressed in Patients with Psoriasis Vulgaris and Protects Mice from Psoriasis-like Skin Inflammation. Immunology 2015, 145, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhang, S.; Zhou, Y.; Lai, S.; Chen, Y.; Geng, Y.; Wang, J. Curcumin Alleviates Imiquimod-Induced Psoriasis in Progranulin-Knockout Mice. Eur. J. Pharmacol. 2021, 909, 174431. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, W.; Zhang, Y.; Zeng, Y. Curcumin Alleviates Imiquimod-induced Psoriasis-like Inflammation and Regulates Gut Microbiota of Mice. Immun. Inflamm. Dis. 2023, 11, e967. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Li, F.; Xiao, H. The Nutraceutical Bioavailability Classification Scheme: Classifying Nutraceuticals According to Factors Limiting Their Oral Bioavailability. Annu. Rev. Food Sci. 2015, 6, 299–327. [Google Scholar] [CrossRef]
- Kurd, S.K.; Smith, N.; VanVoorhees, A.; Troxel, A.B.; Badmaev, V.; Seykora, J.T.; Gelfand, J.M. Oral Curcumin in the Treatment of Moderate to Severe Psoriasis Vulgaris: A Prospective Clinical Trial. J. Am. Acad. Dermatol. 2008, 58, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulsion Loaded Polymeric Hydrogel for Topical Delivery of Curcumin in Psoriasis. J. Drug Deliv. Sci. Technol. 2020, 59, 101847. [Google Scholar] [CrossRef]
- Reena, K.; Mittal, S.; Faizan, M.; Jahan, I.; Rahman, Y.; Khan, R.; Singh, L.; Alhalmi, A.; Noman, O.M.; Alahdab, A. Enhancement of Curcumin’s Anti-Psoriatic Efficacy via Formulation into Tea Tree Oil-Based Emulgel. Gels 2023, 9, 973. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, Y.; Yang, C.; Li, F.; Qiu, B.; Ding, W. Enhanced Transdermal Efficiency of Curcumin-Loaded Peptide-Modified Liposomes for Highly Effective Antipsoriatic Therapy. J. Mater. Chem. B 2021, 9, 4846–4856. [Google Scholar] [CrossRef]
- Sharma, M.; Dhiman, N.; Singh, P.; Sharma, R.; Sharma, R.B.; Arora, V.; Arora, S. Gel Incorporated Lipid Nanoparticles for the Treatment of Psoriasis. Mater. Today Proc. 2022, 48, 1690–1701. [Google Scholar] [CrossRef]
- Iriventi, P.; Gupta, N.V.; Osmani, R.A.M.; Balamuralidhara, V. Design & Development of Nanosponge Loaded Topical Gel of Curcumin and Caffeine Mixture for Augmented Treatment of Psoriasis. Daru 2020, 28, 489–506. [Google Scholar] [CrossRef]
- Kumar, B.; Sahoo, P.K. Augmented Transdermal Delivery of Curcumin for the Effective Management of Plaque Psoriasis—Design, Formulation, Characterisation, and In Vivo Studies. AAPS PharmSciTech 2023, 24, 134. [Google Scholar] [CrossRef] [PubMed]
- Hespeler, D.; Knoth, D.; Keck, C.M.; Müller, R.H.; Pyo, S.M. smartPearls® for Dermal Bioavailability Enhancement—Long-Term Stabilization of Suspensions by Viscoelasticity. Int. J. Pharm. 2019, 562, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Lin, J.; Yang, C.; Wu, C.; He, J.; Chen, Z.; Yang, Q.; Chen, J.; Zheng, G.; Lv, L.; et al. Enhanced Penetration and Anti-Psoriatic Efficacy of Curcumin by Improved smartPearls Technology with the Addition of Glycyrrhizic Acid. Int. J. Pharm. 2020, 578, 119101. [Google Scholar] [CrossRef] [PubMed]
- Antiga, E.; Bonciolini, V.; Volpi, W.; Del Bianco, E.; Caproni, M. Oral Curcumin (Meriva) Is Effective as an Adjuvant Treatment and Is Able to Reduce IL-22 Serum Levels in Patients with Psoriasis Vulgaris. BioMed Res. Int. 2015, 2015, e283634. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Bergonzi, M.C.; Isacchi, B.; Antiga, E.; Caproni, M. Curcumin Nanoparticles Potentiate Therapeutic Effectiveness of Acitrein in Moderate-to-Severe Psoriasis Patients and Control Serum Cholesterol Levels. J. Pharm. Pharmacol. 2018, 70, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Sobolewski, P.; Maślińska, M.; Wieczorek, M.; Łagun, Z.; Malewska, A.; Roszkiewicz, M.; Nitskovich, R.; Szymańska, E.; Walecka, I. Systemic Sclerosis—Multidisciplinary Disease: Clinical Features and Treatment. Reumatologia 2019, 57, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.P.; Khanna, D. Systemic Sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Bairkdar, M.; Rossides, M.; Westerlind, H.; Hesselstrand, R.; Arkema, E.V.; Holmqvist, M. Incidence and Prevalence of Systemic Sclerosis Globally: A Comprehensive Systematic Review and Meta-Analysis. Rheumatology 2021, 60, 3121–3133. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Chen, M.-J.; Yu, Y.-M.; Ko, S.-Y.; Chang, C.-C. Suppression of TGF-Β1/SMAD Pathway and Extracellular Matrix Production in Primary Keloid Fibroblasts by Curcuminoids: Its Potential Therapeutic Use in the Chemoprevention of Keloid. Arch. Dermatol. Res. 2010, 302, 717–724. [Google Scholar] [CrossRef]
- Ryu, H.-W.; Kim, S.-P.; Lee, K.-S.; Cho, J.-W. Curcumin induced decreased expression of type I collagen in human skin fibroblast through down-regulation of Smad2/3 expressions. Korean J. Dermatol. 2012, 50, 1–7. [Google Scholar]
- Song, K.; Peng, S.; Sun, Z.; Li, H.; Yang, R. Curcumin Suppresses TGF-β Signaling by Inhibition of TGIF Degradation in Scleroderma Fibroblasts. Biochem. Biophys. Res. Commun. 2011, 411, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, S.A.; Gaidarova, S.; Saitta, B.; Sandorfi, N.; Herrich, D.J.; Rosenbloom, J.C.; Kucich, U.; Abrams, W.R.; Rosenbloom, J. Role of Protein Kinase C-δ in the Regulation of Collagen Gene Expression in Scleroderma Fibroblasts. J. Clin. Investig. 2001, 108, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Wermuth, P.J.; Addya, S.; Jimenez, S.A. Effect of Protein Kinase C Delta (PKC-δ) Inhibition on the Transcriptome of Normal and Systemic Sclerosis Human Dermal Fibroblasts in Vitro. PLoS ONE 2011, 6, e27110. [Google Scholar] [CrossRef]
- Conboy, L.; Foley, A.G.; O’Boyle, N.M.; Lawlor, M.; Gallagher, H.C.; Murphy, K.J.; Regan, C.M. Curcumin-Induced Degradation of PKCδ Is Associated with Enhanced Dentate NCAM PSA Expression and Spatial Learning in Adult and Aged Wistar Rats. Biochem. Pharmacol. 2009, 77, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Tourkina, E.; Hoffman, S.; Fenton II, J.W.; Lipsitz, S.; Silver, R.M.; Ludwicka-Bradley, A. Depletion of Protein Kinase Cε in Normal and Scleroderma Lung Fibroblasts Has Opposite Effects on Tenascin Expression. Arthritis Rheum. 2001, 44, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Tourkina, E.; Gooz, P.; Oates, J.C.; Ludwicka-Bradley, A.; Silver, R.M.; Hoffman, S. Curcumin-Induced Apoptosis in Scleroderma Lung Fibroblasts. Am. J. Respir. Cell Mol. Biol. 2004, 31, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sethi, G.S.; Naura, A.S. Curcumin Ameliorates Ovalbumin-Induced Atopic Dermatitis and Blocks the Progression of Atopic March in Mice. Inflammation 2020, 43, 358–369. [Google Scholar] [CrossRef]
- Brunner, P.M.; Guttman-Yassky, E.; Leung, D.Y.M. The Immunology of AD and Its Reversibility with Broad Spectrum and Targeted Therapies. J. Allergy Clin. Immunol. 2017, 139, S65–S76. [Google Scholar] [CrossRef]
- Furue, M.; Chiba, T.; Tsuji, G.; Ulzii, D.; Kido-Nakahara, M.; Nakahara, T.; Kadono, T. Atopic Dermatitis: Immune Deviation, Barrier Dysfunction, IgE Autoreactivity and New Therapies. Allergol. Int. 2017, 66, 398–403. [Google Scholar] [CrossRef]
- Tran, M.M.; Lefebvre, D.L.; Dharma, C.; Dai, D.; Lou, W.Y.W.; Subbarao, P.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Sears, M.R.; et al. Predicting the Atopic March: Results from the Canadian Healthy Infant Longitudinal Development Study. J. Allergy Clin. Immunol. 2018, 141, 601–607.e8. [Google Scholar] [CrossRef]
- Moon, P.-D.; Jeong, H.-J.; Kim, H.-M. Down-Regulation of Thymic Stromal Lymphopoietin by Curcumin. Pharmacol. Rep. 2013, 65, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Rawal, R.C.; Shah, B.J.; Jayaraaman, A.M.; Jaiswal, V. Clinical Evaluation of an Indian Polyherbal Topical Formulation in the Management of Eczema. J. Altern. Complement. Med. 2009, 15, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Calapai, G.; Miroddi, M.; Minciullo, P.L.; Caputi, A.P.; Gangemi, S.; Schmidt, R.J. Contact Dermatitis as an Adverse Reaction to Some Topically Used European Herbal Medicinal Products—Part 1: Achillea Millefolium-Curcuma longa. Contact Dermat. 2014, 71, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Villafuerte, L.; Clores, K.H.M. Contact Dermatitis Caused by Turmeric in a Massage Oil. Contact Dermat. 2016, 75, 52–53. [Google Scholar] [CrossRef] [PubMed]
- Liddle, M.; Hull, C.; Liu, C.; Powell, D. Contact Urticaria from Curcumin. Dermatitis 2006, 17, 196–197. [Google Scholar] [CrossRef]
- Vollono, L.; Falconi, M.; Gaziano, R.; Iacovelli, F.; Dika, E.; Terracciano, C.; Bianchi, L.; Campione, E. Potential of Curcumin in Skin Disorders. Nutrients 2019, 11, 2169. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.; Jeon, B.-S.; Jang, W.-S.; Lee, S.-J.; Son, Y.; Rhim, K.-J.; Lee, S.I.; Lee, S.-S. Therapeutic Effect of Topical Application of Curcumin during Treatment of Radiation Burns in a Mini-Pig Model. J. Vet. Sci. 2016, 17, 435–444. [Google Scholar] [CrossRef]
- Ryan, J.L.; Heckler, C.E.; Ling, M.; Katz, A.; Williams, J.P.; Pentland, A.P.; Morrow, G.R. Curcumin for Radiation Dermatitis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Thirty Breast Cancer Patients. Radiat. Res. 2013, 180, 34–43. [Google Scholar] [CrossRef]
- Panahi, Y.; Sahebkar, A.; Amiri, M.; Davoudi, S.M.; Beiraghdar, F.; Hoseininejad, S.L.; Kolivand, M. Improvement of Sulphur Mustard-Induced Chronic Pruritus, Quality of Life and Antioxidant Status by Curcumin: Results of a Randomised, Double-Blind, Placebo-Controlled Trial. Br. J. Nutr. 2012, 108, 1272–1279. [Google Scholar] [CrossRef]
- Scontre, V.A.; Martins, J.C.; de Melo Sette, C.V.; Mutti, H.; Cubero, D.; Fonseca, F.; Del Giglio, A. Curcuma longa (Turmeric) for Prevention of Capecitabine-Induced Hand-Foot Syndrome: A Pilot Study. J. Diet. Suppl. 2018, 15, 606–612. [Google Scholar] [CrossRef]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound Microbiology and Associated Approaches to Wound Management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [PubMed]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a Wound Healing Agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Enoch, S.; Grey, J.E.; Harding, K.G. Recent Advances and Emerging Treatments. BMJ 2006, 332, 962–965. [Google Scholar] [CrossRef]
- Gonzalez, A.C.d.O.; Costa, T.F.; Andrade, Z.d.A.; Medrado, A.R.A.P. Wound Healing—A Literature Review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Zhou, P.; Zhou, H.; Shu, J.; Fu, S.; Yang, Z. Skin Wound Healing Promoted by Novel Curcumin-Loaded Micelle Hydrogel. Ann. Transl. Med. 2021, 9, 1152. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Al-Qudaihi, A.; Alaseel, S.E.; Fita, I.Z.; Khalid, M.S.; Pottoo, F.H. Preparation of a Novel Curcumin Nanoemulsion by Ultrasonication and Its Comparative Effects in Wound Healing and the Treatment of Inflammation. RSC Adv. 2019, 9, 20192–20206. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.; Li, Y.; Pang, X.; Wang, B.; Wu, Z.; Lu, Y.; Xiong, K.; Zhao, L.; Zhou, P.; Fu, S. Curcumin Nanoparticles Incorporated in PVA/Collagen Composite Films Promote Wound Healing. Drug Deliv. 2020, 27, 1676–1685. [Google Scholar] [CrossRef]
- Mohanty, C.; Pradhan, J. A Human Epidermal Growth Factor-Curcumin Bandage Bioconjugate Loaded with Mesenchymal Stem Cell for in Vivo Diabetic Wound Healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110751. [Google Scholar] [CrossRef]
- Shakiba, M.; Sheikhi, M.; Pahnavar, Z.; Tajiki, A.; Bigham, A.; Foroozandeh, A.; Darvishan, S.; Pourmadadi, M.; Emadi, H.; Rezatabar, J.; et al. Development of an Antibacterial and Antioxidative Nanofibrous Membrane Using Curcumin-Loaded Halloysite Nanotubes for Smart Wound Healing: In Vitro and In Vivo Studies. Int. J. Pharm. 2023, 642, 123207. [Google Scholar] [CrossRef]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Li, H.-Y.; Yang, M.; Li, Z.; Meng, Z. Curcumin Inhibits Angiotensin II-Induced Inflammation and Proliferation of Rat Vascular Smooth Muscle Cells by Elevating PPAR-γ Activity and Reducing Oxidative Stress. Int. J. Mol. Med. 2017, 39, 1307–1316. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Wu, J.; Bai, B.; Chen, H.; Xiao, Z.; Chen, L.; Zhao, Y.; Lum, H.; Wang, Y.; et al. New MD2 Inhibitors Derived from Curcumin with Improved Anti-Inflammatory Activity. Eur. J. Med. Chem. 2018, 148, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Fereydouni, N.; Darroudi, M.; Movaffagh, J.; Shahroodi, A.; Butler, A.E.; Ganjali, S.; Sahebkar, A. Curcumin Nanofibers for the Purpose of Wound Healing. J. Cell. Physiol. 2019, 234, 5537–5554. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, D.; Ahmed, M.R.; Gomathi, K.; Chitra, K.; Sehgal, P.K.; Jayakumar, R. Dermal Wound Healing Processes with Curcumin Incorporated Collagen Films. Biomaterials 2004, 25, 1911–1917. [Google Scholar] [CrossRef] [PubMed]
- Kant, V.; Gopal, A.; Pathak, N.N.; Kumar, P.; Tandan, S.K.; Kumar, D. Antioxidant and Anti-Inflammatory Potential of Curcumin Accelerated the Cutaneous Wound Healing in Streptozotocin-Induced Diabetic Rats. Int. Immunopharmacol. 2014, 20, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dai, C.; Wang, Z.; Chen, W.; Liu, J.; Zhuo, R.; Yu, A.; Huang, S. A Novel Curcumin-Loaded Composite Dressing Facilitates Wound Healing Due to Its Natural Antioxidant Effect. Drug Des. Dev. Ther. 2019, 13, 3269–3280. [Google Scholar] [CrossRef] [PubMed]
- Bielefeld, K.A.; Amini-Nik, S.; Alman, B.A. Cutaneous Wound Healing: Recruiting Developmental Pathways for Regeneration. Cell Mol. Life Sci. 2013, 70, 2059–2081. [Google Scholar] [CrossRef] [PubMed]
- Diller, R.B.; Tabor, A.J. The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimetics 2022, 7, 87. [Google Scholar] [CrossRef]
- Yen, Y.-H.; Pu, C.-M.; Liu, C.-W.; Chen, Y.-C.; Chen, Y.-C.; Liang, C.-J.; Hsieh, J.-H.; Huang, H.-F.; Chen, Y.-L. Curcumin Accelerates Cutaneous Wound Healing via Multiple Biological Actions: The Involvement of TNF-α, MMP-9, α-SMA, and Collagen. Int. Wound J. 2018, 15, 605–617. [Google Scholar] [CrossRef]
- Chen, K.; Pan, H.; Ji, D.; Li, Y.; Duan, H.; Pan, W. Curcumin-Loaded Sandwich-like Nanofibrous Membrane Prepared by Electrospinning Technology as Wound Dressing for Accelerate Wound Healing. Mater. Sci. Eng. C 2021, 127, 112245. [Google Scholar] [CrossRef]
- Rujirachotiwat, A.; Suttamanatwong, S. Curcumin Promotes Collagen Type I, Keratinocyte Growth Factor-1, and Epidermal Growth Factor Receptor Expressions in the In Vitro Wound Healing Model of Human Gingival Fibroblasts. Eur. J. Dent. 2021, 15, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Huang, C.; Huang, H.; Zhao, Y.; Khan, M.R.U.; Zhao, H.; Huang, L. Antibacterial Mechanism of Curcumin: A Review. Chem. Biodivers. 2020, 17, e2000171. [Google Scholar] [CrossRef] [PubMed]
- Paolillo, F.R.; Rodrigues, P.G.S.; Bagnato, V.S.; Alves, F.; Pires, L.; Corazza, A.V. The Effect of Combined Curcumin-Mediated Photodynamic Therapy and Artificial Skin on Staphylococcus aureus–Infected Wounds in Rats. Lasers Med. Sci. 2021, 36, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, I.d.P.; Pinto, J.G.; Souza, B.M.N.; Miñán, A.G.; Ferreira-Strixino, J. Antimicrobial Photodynamic Therapy with Curcumin on Methicillin-Resistant Staphylococcus aureus Biofilm. Photodiagn. Photodyn. Ther. 2022, 37, 102729. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.D.; Alves, F.; Buzza, H.H.; Bagnato, V.S. Photodisinfection of material surfaces and bacterial skin infections by a detergent loaded with curcumin. Photodiagnosis Photodyn. Ther. 2022, 39, 103021. [Google Scholar] [CrossRef] [PubMed]
- Bugli, F.; Cacaci, M.; Palmieri, V.; Di Santo, R.; Torelli, R.; Ciasca, G.; Di Vito, M.; Vitali, A.; Conti, C.; Sanguinetti, M.; et al. Curcumin-Loaded Graphene Oxide Flakes as an Effective Antibacterial System against Methicillin-Resistant Staphylococcus aureus. Interface Focus 2018, 8, 20170059. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, F.; Khan, A.U.; Misba, L.; Akhtar, K.; Ali, A. Antimicrobial and Antibiofilm Photodynamic Therapy against Vancomycin Resistant Staphylococcus aureus (VRSA) Induced Infection In Vitro and In Vivo. Eur. J. Pharm. Biopharm. 2021, 160, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Almeida, P.P.; Pereira, Í.S.; Rodrigues, K.B.; Leal, L.S.; Marques, A.S.; Rosa, L.P.; da Silva, F.C.; da Silva, R.A.A. Photodynamic Therapy Controls of Staphylococcus aureus Intradermal Infection in Mice. Lasers Med. Sci. 2017, 32, 1337–1342. [Google Scholar] [CrossRef]
- Yulinah Sukandar, E.; Fisheri Kurniati, N.; Puspatriani, K.; Puspita Adityas, H. Antibacterial Activity of Curcumin in Combination with Tetracycline against Staphylococcus aureus by Disruption of Cell Wall. Res. J. Med. Plants 2018, 12, 1–8. [Google Scholar] [CrossRef]
- Elkhateeb, O.; Badawy, M.E.I.; Tohamy, H.G.; Abou-Ahmed, H.; El-Kammar, M.; Elkhenany, H. Curcumin-Infused Nanostructured Lipid Carriers: A Promising Strategy for Enhancing Skin Regeneration and Combating Microbial Infection. BMC Vet. Res. 2023, 19, 206. [Google Scholar] [CrossRef]
- Taghavifar, S.; Afroughi, F.; Keyvan, M. Curcumin Nanoparticles Improved Diabetic Wounds Infected With Methicillin-Resistant Staphylococcus aureus Sensitized With HAMLET. Int. J. Low. Extrem. Wounds 2022, 21, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Badali, H.; Wiederhold, N.P. Antifungal Resistance Testing and Implications for Management. Curr. Fungal Infect. Rep. 2019, 13, 274–283. [Google Scholar] [CrossRef]
- Apisariyakul, A.; Vanittanakom, N.; Buddhasukh, D. Antifungal Activity of Turmeric Oil Extracted from Curcuma longa (Zingiberaceae). J. Ethnopharmacol. 1995, 49, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Ghadi, Z.; Vaezi, A.; Ahangarkani, F.; Ilkit, M.; Ebrahimnejad, P.; Badali, H. Potent In Vitro Activity of Curcumin and Quercetin Co-Encapsulated in Nanovesicles without Hyaluronan against Aspergillus and Candida Isolates. J. Mycol. Méd. 2020, 30, 101014. [Google Scholar] [CrossRef] [PubMed]
- Brasch, J.; Freitag-Wolf, S.; Beck-Jendroschek, V.; Huber, M. Inhibition of Dermatophytes by Photodynamic Treatment with Curcumin. Med. Mycol. 2017, 55, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Brasch, J.; Beck-Jendroschek, V.; Mahn, V. Photochemical Inhibition of Trichophyton Rubrum by Different Compoundings of Curcumin. Mycoses 2018, 61, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Mishra, L.; Gupta, S. Fluconazole and Curcumin Loaded Nanoemulsion Against Multiple Drug Resistance Dermatophytes. Biomed. Pharmacol. J. 2021, 14, 2085–2094. [Google Scholar] [CrossRef]
- Al Fatease, A.; Alqahtani, A.; Khan, B.A.; Mohamed, J.M.M.; Farhana, S.A. Preparation and Characterization of a Curcumin Nanoemulsion Gel for the Effective Treatment of Mycoses. Sci. Rep. 2023, 13, 22730. [Google Scholar] [CrossRef]
- Anwar, S.K.; Elmonaem, S.N.A.; Moussa, E.; Aboulela, A.G.; Essawy, M.M. Curcumin Nanoparticles: The Topical Antimycotic Suspension Treating Oral Candidiasis. Odontology 2023, 111, 350–359. [Google Scholar] [CrossRef]
- Alonso, L.; Dorta, M.L.; Alonso, A. Ivermectin and Curcumin Cause Plasma Membrane Rigidity in Leishmania amazonensis Due to Oxidative Stress. Biochim. Biophys. Acta (BBA) Biomembr. 2022, 1864, 183977. [Google Scholar] [CrossRef]
- Pereira, A.H.C.; Marcolino, L.M.C.; Pinto, J.G.; Ferreira-Strixino, J. Evaluation of the Photodynamic Therapy with Curcumin on L. braziliensis and L. major Amastigotes. Antibiotics 2021, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.M.; Hadighi, R.; Najm, M.; Alipour, M.; Hasanpour, H.; Vosoogh, M.; Vosough, A.; Hajizadeh, M.; Badirzadeh, A. The Therapeutic Effects of Curcumin-Coated Gold Nanoparticle Against Leishmania Major Causative Agent of Zoonotic Cutaneous Leishmaniasis (ZCL): An In Vitro and In Vivo Study. Curr. Microbiol. 2023, 80, 104. [Google Scholar] [CrossRef] [PubMed]
- Saeed, B.Q.; Hussain, K.; Akbar, N.; Khan, H.; Siddiqui, R.; Shah, R.M.; Khan, N.A. Nanovesicles Containing Curcumin Hold Promise in the Development of New Formulations of Anti-Acanthamoebic Agents. Mol. Biochem. Parasitol. 2022, 247, 111430. [Google Scholar] [CrossRef] [PubMed]
- Hezarjaribi, H.Z.; Mollarostami, F.; Ebrahimnejad, P.; Esboei, B.R.; Fakhar, M.; Sadeghi-Ghadi, Z. Promising Potent in Vitro Activity of Curcumin and Quercetin Nano-Niosomes against Trichomonas Vaginalis. Ann. Parasitol. 2022, 68, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Mallo, N.; Lamas, J.; Sueiro, R.A.; Leiro, J.M. Molecular Targets Implicated in the Antiparasitic and Anti-Inflammatory Activity of the Phytochemical Curcumin in Trichomoniasis. Molecules 2020, 25, 5321. [Google Scholar] [CrossRef] [PubMed]
- Šudomová, M.; Hassan, S.T.S. Nutraceutical Curcumin with Promising Protection against Herpesvirus Infections and Their Associated Inflammation: Mechanisms and Pathways. Microorganisms 2021, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Kutluay, S.B.; Doroghazi, J.; Roemer, M.E.; Triezenberg, S.J. Curcumin Inhibits Herpes Simplex Virus Immediate-Early Gene Expression by a Mechanism Independent of P300/CBP Histone Acetyltransferase Activity. Virology 2008, 373, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.H.; Nazli, A.; Dizzell, S.E.; Mueller, K.; Kaushic, C. The Anti-Inflammatory Activity of Curcumin Protects the Genital Mucosal Epithelial Barrier from Disruption and Blocks Replication of HIV-1 and HSV-2. PLoS ONE 2015, 10, e0124903. [Google Scholar] [CrossRef]
- Flores, D.J.; Lee, L.H.; Adams, S.D. Inhibition of Curcumin-Treated Herpes Simplex Virus 1 and 2 In Vero Cells. Adv. Microbiol. 2016, 6, 276–287. [Google Scholar] [CrossRef]
- Vitali, D.; Bagri, P.; Wessels, J.M.; Arora, M.; Ganugula, R.; Parikh, A.; Mandur, T.; Felker, A.; Garg, S.; Kumar, M.N.V.R.; et al. Curcumin Can Decrease Tissue Inflammation and the Severity of HSV-2 Infection in the Female Reproductive Mucosa. Int. J. Mol. Sci. 2020, 21, 337. [Google Scholar] [CrossRef]
- El-Halim, S.M.A.; Mamdouh, M.A.; El-Haddad, A.E.; Soliman, S.M. Fabrication of Anti-HSV-1 Curcumin Stabilized Nanostructured Proniosomal Gel: Molecular Docking Studies on Thymidine Kinase Proteins. Sci. Pharm. 2020, 88, 9. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Sharma, A.; Maheshwari, R.K. Beneficial role of curcumin in skin diseases. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Aggarwal, B.B., Surh, Y.-J., Shishodia, S., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 2007; pp. 343–357. ISBN 978-0-387-46401-5. [Google Scholar]
- Olczyk, K.; Partyka, R.; Misiło, A.; Zając, K. VEGF and Its Receptors—Formation, Structure, and Role in the Organism. J. Educ. Health Sport 2023, 21, 212–226. [Google Scholar] [CrossRef]
- Wang, T.; Chen, J. Effects of Curcumin on Vessel Formation Insight into the Pro- and Antiangiogenesis of Curcumin. Evid.-Based Complement. Altern. Med. 2019, 2019, 1390795. [Google Scholar] [CrossRef] [PubMed]
- Kant, V.; Gopal, A.; Kumar, D.; Pathak, N.N.; Ram, M.; Jangir, B.L.; Tandan, S.K.; Kumar, D. Curcumin-Induced Angiogenesis Hastens Wound Healing in Diabetic Rats. J. Surg. Res. 2015, 193, 978–988. [Google Scholar] [CrossRef]
- Tu, Z.; Jiang, X.; Li, Y.; Yang, S.; Lin, D.; Shi, Y.; Mao, C.; Zhang, X.; Lin, C. Curcumin Promotes the Survival of Ischemic Random Skin Flaps via Autophagy. Am. J. Transl. Res. 2021, 13, 1337–1351. [Google Scholar] [PubMed]
- Abusnina, A.; Keravis, T.; Zhou, Q.; Justiniano, H.; Lobstein, A.; Lugnier, C. Tumour Growth Inhibition and Anti-Angiogenic Effects Using Curcumin Correspond to Combined PDE2 and PDE4 Inhibition. Thromb. Haemost. 2015, 113, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Zhai, Y.; He, Z.; Wang, Q.; Sun, L.; Sun, T.; Lv, L.; Li, Y.; Yang, J.; Lv, D.; et al. Water-Responsive Gel Extends Drug Retention and Facilitates Skin Penetration for Curcumin Topical Delivery against Psoriasis. Asian J. Pharm. Sci. 2023, 18, 100782. [Google Scholar] [CrossRef] [PubMed]
- Hipólito-Reis, M.; Neto, A.C.; Neves, D. Impact of Curcumin, Quercetin, or Resveratrol on the Pathophysiology of Endometriosis: A Systematic Review. Phytother. Res. 2022, 36, 2416–2433. [Google Scholar] [CrossRef]
- Shalaby, E.S.; Aboutaleb, S.; Ismail, S.A.; Yassen, N.N.; Sedik, A.A. Chitosan Tamarind-Based Nanoparticles as a Promising Approach for Topical Application of Curcumin Intended for Burn Healing: In Vitro and In Vivo Study. J. Drug Target. 2023, 31, 1081–1097. [Google Scholar] [CrossRef]
- Osika, G.; Wesołowska, A. Non-Surgical Methods for Delaying Skin Aging Processes. Farm. Pol. 2020, 76, 110–117. [Google Scholar] [CrossRef]
- Sharma, A.; Kuhad, A.; Bhandari, R. Novel Nanotechnological Approaches for Treatment of Skin-Aging. J. Tissue Viability 2022, 31, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Tavakol, S.; Zare, S.; Hoveizi, E.; Tavakol, B.; Rezayat, S.M. The Impact of the Particle Size of Curcumin Nanocarriers and the Ethanol on Beta_1-Integrin Overexpression in Fibroblasts: A Regenerative Pharmaceutical Approach in Skin Repair and Anti-Aging Formulations. Daru 2019, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The Role of Curcumin in Aging and Senescence: Molecular Mechanisms. Biomed. Pharmacother. 2021, 134, 111119. [Google Scholar] [CrossRef] [PubMed]
- El Hosary, R.; Teaima, M.H.; El-Nabarawi, M.; Yousry, Y.; Eltahan, M.; Bakr, A.; Aboelela, H.; Abdelmonem, R.; Nassif, R.M. Topical Delivery of Extracted Curcumin as Curcumin Loaded Spanlastics Anti-Aging Gel: Optimization Using Experimental Design and Ex-Vivo Evaluation. Saudi Pharm. J. 2024, 32, 101912. [Google Scholar] [CrossRef] [PubMed]
- Sghier, K.; Mur, M.; Veiga, F.; Paiva-Santos, A.C.; Pires, P.C. Novel Therapeutic Hybrid Systems Using Hydrogels and Nanotechnology: A Focus on Nanoemulgels for the Treatment of Skin Diseases. Gels 2024, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- de Souza Silva, F.K.; Costa-Orlandi, C.B.; Fernandes, M.A.; Pegorin Brasil, G.S.; Mussagy, C.U.; Scontri, M.; da Sasaki, J.C.; de Sousa Abreu, A.P.; Guerra, N.B.; Floriano, J.F.; et al. Biocompatible Anti-Aging Face Mask Prepared with Curcumin and Natural Rubber with Antioxidant Properties. Int. J. Biol. Macromol. 2023, 242, 124778. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasprzak-Drozd, K.; Niziński, P.; Hawrył, A.; Gancarz, M.; Hawrył, D.; Oliwa, W.; Pałka, M.; Markowska, J.; Oniszczuk, A. Potential of Curcumin in the Management of Skin Diseases. Int. J. Mol. Sci. 2024, 25, 3617. https://doi.org/10.3390/ijms25073617
Kasprzak-Drozd K, Niziński P, Hawrył A, Gancarz M, Hawrył D, Oliwa W, Pałka M, Markowska J, Oniszczuk A. Potential of Curcumin in the Management of Skin Diseases. International Journal of Molecular Sciences. 2024; 25(7):3617. https://doi.org/10.3390/ijms25073617
Chicago/Turabian StyleKasprzak-Drozd, Kamila, Przemysław Niziński, Anna Hawrył, Marek Gancarz, Dominika Hawrył, Weronika Oliwa, Magdalena Pałka, Julia Markowska, and Anna Oniszczuk. 2024. "Potential of Curcumin in the Management of Skin Diseases" International Journal of Molecular Sciences 25, no. 7: 3617. https://doi.org/10.3390/ijms25073617
APA StyleKasprzak-Drozd, K., Niziński, P., Hawrył, A., Gancarz, M., Hawrył, D., Oliwa, W., Pałka, M., Markowska, J., & Oniszczuk, A. (2024). Potential of Curcumin in the Management of Skin Diseases. International Journal of Molecular Sciences, 25(7), 3617. https://doi.org/10.3390/ijms25073617