Nervonic Acid Synthesis Substrates as Essential Components in Profiled Lipid Supplementation for More Effective Central Nervous System Regeneration
Abstract
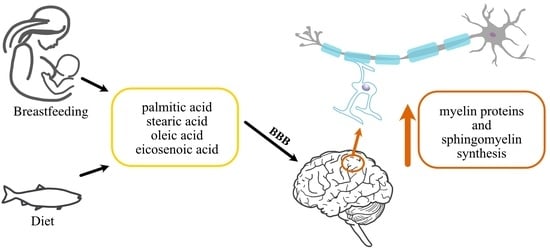
:1. Introduction
2. How Are the Lipid Components Necessary for Myelin Formation Supplied?
3. Substrates for NA Synthesis Proved to Be Crucial in the Remyelination Process
4. Effective Remyelination Based on the Properly Profiled Lipid Intake
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, D.; Huang, Y.; Shi, Z.; Li, J.; Zhang, Y.; Wang, K.; Smith, A.D.; Gong, Y.; Gao, Y. Demyelinating processes in aging and stroke in the central nervous system and the prospect of treatment strategy. CNS Neurosci. Ther. 2020, 26, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Palavicini, J.P.; Wang, C.; Chen, L.; Ahmar, S.; Higuera, J.D.; Dupree, J.L.; Han, X. Novel molecular insights into the critical role of sulfatide in myelin maintenance/function. J. Neurochem. 2016, 139, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Salzer, J.L. Schwann cell myelination. Cold Spring Harb. Perspect. Biol. 2015, 7, a020529. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, H.; Baron, W.; Hoekstra, D.; Kahya, N. Oligodendroglial membrane dynamics in relation to myelin biogenesis. Cell Mol. Life Sci. 2016, 73, 3291–3310. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.S. Stability of the myelin membrane. Science 1965, 147, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Chrast, R.; Saher, G.; Nave, K.A.; Verheijen, M.H. Lipid metabolism in myelinating glial cells: Lessons from human inherited disorders and mouse models. J. Lipid Res. 2011, 52, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Jackman, N.; Ishii, A.; Bansa, R. Oligodendrocyte development and myelin biogenesis: Parsing out the roles of glycosphingolipids. Physiol. Bethesda 2009, 24, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Norton, W.T.; Poduslo, S.E. Myelination in rat brain: Changes in myelin composition during brain maturation. J. Neurochem. 1973, 21, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Prineas, J.W.; Connell, F. Remyelination in multiple sclerosis. Ann. Neurol. 1979, 5, 22–31. [Google Scholar] [CrossRef]
- Reeves, T.M.; Smith, T.L.; Williamson, J.C.; Phillips, L.L. Unmyelinated axons show selective rostrocaudal pathology in the corpus callosum after traumatic brain injury. J. Neuropathol. Exp. Neurol. 2012, 71, 198Y210. [Google Scholar] [CrossRef]
- Hofmann, K.; Rodriguez-Rodriguez, R.; Gaebler, A.; Casals, N.; Scheller, A.; Kuerschner, L. Astrocytes and oligodendrocytes in grey and white matter regions of the brain metabolize fatty acids. Sci. Rep. 2017, 7, 10779. [Google Scholar] [CrossRef] [PubMed]
- Duncan, I.D.; Radcliff, A.B. Inherited and acquired disorders of myelin: The underlying myelin pathology. Exp. Neurol. 2016, 283, 452–475. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Alfvén, G.; Blennow, M.; Trygg, M.; Zetterström, R. Long-chain polyunsaturated fatty acids in human milk and brain growth during early infancy. Acta Paediatr. 2000, 89, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Lewkowicz, N.; Piątek, P.; Namiecińska, M.; Domowicz, M.; Bonikowski, R.; Szemraj, J.; Przygodzka, P.; Stasiołek, M.; Lewkowicz, P. Naturally Occurring Nervonic Acid Ester Improves Myelin Synthesis by Human Oligodendrocytes. Cells 2019, 8, 786. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Griel, A.E.; Psota, T.L.; Gebauer, S.K.; Zhang, J.; Etherton, T.D. Dietary stearic acid and risk of cardiovascular disease: Intake, sources, digestion, and absorption. Lipids 2005, 40, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef]
- Voskuhl, R.R.; Itoh, N.; Tassoni, A.; Matsukawa, M.A.; Ren, E.; Tse, V.; Jang, E.; Suen, T.T.; Itoh, Y. Gene expression in oligodendrocytes during remyelination reveals cholesterol homeostasis as a therapeutic target in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2019, 116, 10130–10139. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.W.; Hatch, G.M. Fatty acid transport into the brain: Of fatty acid fables and lipid tails. Prostaglandins Leukot. Essent. Fatty Acids. 2011, 85, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Takechi, R.; Pallebage-Gamarallage, M.M.; Lam, V.; Giles, C.; Mamo, J.C. Aging-related changes in blood-brain barrier integrity and the effect of dietary fat. Neurodegener Dis. 2013, 12, 125–135. [Google Scholar] [CrossRef]
- Sherry, D.M.; Hopiavuori, B.R.; Stiles, M.A.; Rahman, N.S.; Ozan, K.G.; Deak, F.; Agbaga, M.P.; Anderson, R.E. Distribution of ELOVL4 in the Developing and Adult Mouse Brain. Front. Neuroanat. 2017, 11, 38. [Google Scholar] [CrossRef]
- Jump, D.B. Mammalian fatty acid elongases. Methods Mol. Biol. 2009, 579, 375–389. [Google Scholar] [CrossRef]
- Bogie, J.F.J.; Grajchen, E.; Wouters, E.; Corrales, A.G.; Dierckx, T.; Vanherle, S.; Mailleux, J.; Gervois, P.; Wolfs, E.; Dehairs, J.; et al. Stearoyl-CoA desaturase-1 impairs the reparative properties of macrophages and microglia in the brain. J. Exp. Med. 2020, 217, e20191660. [Google Scholar] [CrossRef]
- Wahrle, S.E.; Jiang, H.; Parsadanian, M.; Legleiter, J.; Han, X.; Fryer, J.D.; Kowalewski, T.; Holtzman, D.M. ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J. Biol. Chem. 2004, 279, 40987–40993. [Google Scholar] [CrossRef] [PubMed]
- Garcia Corrales, A.V.; Haidar, M.; Bogie, J.F.J.; Hendriks, J.J.A. Fatty Acid Synthesis in Glial Cells of the CNS. Int. J. Mol. Sci. 2021, 22, 8159. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Calle, R.; Konings, S.C.; Frontiñán-Rubio, J.; García-Revilla, J.; Camprubí-Ferrer, L.; Svensson, M.; Martinson, I.; Boza-Serrano, A.; Venero, J.L.; Nielsen, H.M.; et al. APOE in the bullseye of neurodegenerative diseases: Impact of the APOE genotype in Alzheimer’s disease pathology and brain diseases. Mol. Neurodegener. 2022, 17, 62. [Google Scholar] [CrossRef]
- Camargo, N.; Goudriaan, A.; van Deijk, A.F.; Otte, W.M.; Brouwers, J.F.; Lodder, H.; Gutmann, D.H.; Nave, K.A.; Dijkhuizen, R.M.; Mansvelder, H.; et al. Oligodendroglial myelination requires astrocyte-derived lipids. PLoS Biol. 2017, 15, e1002605. [Google Scholar] [CrossRef]
- Stańdo-Retecka, M.; Piątek, P.; Namiecinska, M.; Bonikowski, R.; Lewkowicz, P.; Lewkowicz, N. Clinical and microbiological outcomes of subgingival instrumentation supplemented with high-dose omega-3 polyunsaturated fatty acids in periodontal treatment—A randomized clinical trial. BMC Oral Health 2023, 23, 290. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Yang, X.; Cheng, Y.; Zhang, H.; Xu, X.; Zhou, J.; Chen, H.; Su, M.; Yang, Y.; et al. Human Milk Lipid Profiles around the World: A Systematic Review and Meta-Analysis. Adv. Nutr. 2022, 13, 2519–2536. [Google Scholar] [CrossRef] [PubMed]
- Siziba, L.P.; Lorenz, L.; Brenner, H.; Carr, P.; Stahl, B.; Mank, M.; Marosvölgyi, T.; Decsi, T.; Szabó, É.; Rothenbacher, D.; et al. Changes in human milk fatty acid composition and maternal lifestyle-related factors over a decade: A comparison between the two Ulm Birth Cohort Studies. Br. J. Nutr. 2021, 126, 228–235. [Google Scholar] [CrossRef]
- Koletzko, B.; Thiel, I.; Abiodun, P.O. The fatty acid composition ofhuman milk in Europe and Africa. J. Pediatr. 1992, 120, S62–S70. [Google Scholar] [CrossRef]
- Willard, D.E.; Harmon, S.D.; Kaduce, T.L. Docosahexaenoic acid synthesis from n-3 polyunsaturated fatty acids in differentiated rat brain astrocytes. J. Lipid Res. 2001, 42, 1368–1376. [Google Scholar] [CrossRef]
- Kitson, A.P.; Metherel, A.H.; Chen, C.T.; Domenichiello, A.F.; Trépanier, M.O.; Berger, A.; Bazinet, R.P. Effect of dietary docosahexaenoic acid (DHA) in phospholipids or triglycerides on brain DHA uptake and accretion. J. Nutr. Biochem. 2016, 33, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; Mougan, I. Fatty acid composition of human brain phospholipids during normal development. J. Neurochem. 1998, 71, 2528–2533. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, K.; Xue, T.; Han, J.; Peng, F.; Ding, C.; Lin, F.; Li, J.; Sze, F.T.; Gan, J.; et al. Cognitive improvement effect of nervonic acid and essential fatty acids on rats ingesting Acer truncatum Bunge seed oil revealed by lipidomics approach. Food Funct. 2022, 13, 2475–2490. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Shi, P.; Shen, Z.; Meng, H.; Meng, Z.; Han, X.; Chen, Y.; Fan, W.; Fa, Y.; Yang, C.; et al. High-level production of nervonic acid in the oleaginous yeast Yarrowia lipolytica by systematic metabolic engineering. Commun. Biol. 2023, 6, 1125. [Google Scholar] [CrossRef]
- Chen, J.R.; Hsu, S.F.; Hsu, C.D.; Hwang, L.H.; Yang, S.C. Dietary patterns and blood fatty acid composition in children with attention-deficit hyperactivity disorder in Taiwan. J. Nutr. Biochem. 2004, 15, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, Y.; Deguchi, Y.; Hattori, K.; Yoshida, S.; Goto, Y.I.; Inoue, K.; Kato, T. Nervonic acid level in cerebrospinal fluid is a candidate biomarker for depressive and manic symptoms: A pilot study. Brain Behav. 2021, 11, e02075. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, J.; Yu, X.; Gao, J.M. A mini review of nervonic acid: Source, production, and biological functions. Food Chem. 2019, 301, 125286. [Google Scholar] [CrossRef] [PubMed]
- Velasco, A.; Tabernero, A.; Medina, J.M. Role of oleic acid as a neurotrophic factor is supported in vivo by the expression of GAP-43 subsequent to the activation of SREBP-1 and the up-regulation of stearoyl-CoA desaturase during postnatal development of the brain. Brain Res. 2003, 977, 103–111. [Google Scholar] [CrossRef]
- Amminger, G.P.; Schäfer, M.R.; Klier, C.M.; Slavik, J.M.; Holzer, I.; Holub, M.; Goldstone, S.; Whitford, T.J.; McGorry, P.D.; Berk, M. Decreased nervonic acid levels in erythrocyte membranes predict psychosis in help-seeking ultra-high-risk individuals. Mol. Psychiatry 2012, 17, 1150–1152. [Google Scholar] [CrossRef]
- Kageyama, Y.; Kasahara, T.; Nakamura, T.; Hattori, K.; Deguchi, Y.; Tani, M.; Kuroda, K.; Yoshida, S.; Goto, Y.I.; Inoue, K.; et al. Plasma Nervonic Acid Is a Potential Biomarker for Major Depressive Disorder: A Pilot Study. Int. J. Neuropsychopharmacol. 2018, 21, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Terluk, M.R.; Tieu, J.; Sahasrabudhe, S.A.; Moser, A.; Watkins, P.A.; Raymond, G.V.; Kartha, R.V. Nervonic Acid Attenuates Accumulation of Very Long-Chain Fatty Acids and is a Potential Therapy for Adrenoleukodystrophy. Neurotherapeutics 2022, 19, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Malaisse, W.J.; Portois, L.; Sener, A.; Carpentier, Y.A. Perturbation of 11-eicosenoate metabolism in female diabetic rats. Int. J. Mol. Med. 2008, 22, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Oda, E.; Hatada, K.; Kimura, J.; Aizawa, Y.; Thanikachalam, P.V.; Watanabe, K. Relationships between serum unsaturated fatty acids and coronary risk factors: Negative relations between nervonic acid and obesity-related risk factors. Int. Heart J. 2005, 46, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.N.; Wang, M.X.; Han, J.L.; Feng, C.Y.; Wang, M.; Wang, M.; Sun, J.Y.; Li, N.Y.; Simal-Gandara, J.; Liu, C. Improved colonic inflammation by nervonic acid via inhibition of NF-κB signaling pathway of DSS-induced colitis mice. Phytomedicine 2023, 112, 154702. [Google Scholar] [CrossRef] [PubMed]
- Phung, N.V.; Rong, F.; Xia, W.Y.; Fan, Y.; Li, X.Y.; Wang, S.A.; Li, F.L. Nervonic acid and its sphingolipids: Biological functions and potential food applications. Crit. Rev. Food Sci. Nutr. 2023, 28, 1–20. [Google Scholar] [CrossRef]
- Calder, P. Long-chain fatty acids and inflammation. Proc. Nutr. Soci. 2012, 71, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; Fitz Gerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, P.T.; Jordan, K.; Cederberg, H.; Brohult, J. Some biological actions of alkylglycerols from shark liver oil. J. Altern. Complement. Med. 1998, 4, 87–99. [Google Scholar] [CrossRef]
- Gan, R.; Young, K.; Zerbe, G.; Demoruelle, M.; Weisman, M.; Buckner, J.; Gregersen, P.K.; Mikuls, T.R.; O’Dell, J.R.; Keating, R.M.; et al. Lower omega-3 fatty acids are associated with the presence of anti-cyclic citrullinated peptide autoantibodies in a population at risk for future rheuma-toid arthritis: A nested case-control study. Rheumatology 2015, 55, 367–376. [Google Scholar] [CrossRef]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Edmond, J. Essential polyunsaturated fatty acids and the barrier to the brain. J. Mol. Neurosci. 2001, 16, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wang, X.; Chen, Z.; Landman, N.; Lo, E.H.; Kang, J.X. Gene transfer of Caenorhabditis elegans n-3 fatty acid desaturase inhibits neuronal apoptosis. J. Neurochem. 2002, 82, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, M.; Emond, V.; Chen, C.T.; Julien, C.; Bourasset, F.; Oddo, S. Diffusion of docosahexaenoic and eicosapentaenoic acids through the blood-brain barrier: An in situ cerebral perfusion study. Neurochem. Int. 2009, 55, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.C.; MacIsaac, R.J.; Roberts, L.; Kamel, J.; Craig, J.P.; Busija, L.; Downie, L.E. Omega-3 polyunsaturated fatty acid supplementation for improving peripheral nerve health: Protocol for a systematic review. BMJ Open. 2018, 8, e020804. [Google Scholar] [CrossRef] [PubMed]
- Piątek, P.; Lewkowicz, N.; Michlewska, S.; Wieczorek, M.; Bonikowski, R.; Parchem, K.; Lewkowicz, P.; Namiecinska, M. Natural fish oil improves the differentiation and maturation of oligodendrocyte precursor cells to oligodendrocytes in vitro after interaction with the blood-brain barrier. Front. Immunol. 2022, 13, 932383. [Google Scholar] [CrossRef] [PubMed]
- Sargent, J.R.; Coupland, K.; Wilson, R. Nervonic acid and demyelinating disease. Med. Hypotheses. 1994, 42, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.; Bandaru, V.V.; Calabresi, P.A.; Nath, A.; Haughey, N.J. A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain 2008, 131 Pt 11, 3092–3102. [Google Scholar] [CrossRef]
- Qin, J.; Berdyshev, E.; Goya, J.; Natarajan, V.; Dawson, G. Neurons and oligodendrocytes recycle sphingosine 1-phosphate to ceramide: Significance for apoptosis and multiple sclerosis. J. Biol. Chem. 2010, 285, 14134–14143. [Google Scholar] [CrossRef]
- Schwarz, S.; Leweling, H. Multiple sclerosis and nutrition. Mult. Scler. 2005, 11, 24–32. [Google Scholar] [CrossRef]
- Zhou, L.Q.; Dong, M.H.; Hu, Z.W.; Tang, Y.; Chu, Y.H.; Chen, M.; Yang, S.; Chen, Z.; Wu, L.J.; Wang, W.; et al. Staged suppression of microglial autophagy facilitates regeneration in CNS demyelination by enhancing the production of linoleic acid. Proc. Natl. Acad. Sci. USA 2023, 120, e2209990120. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Shimizu, T.; Ohtsuka, Y.; Yamashiro, Y.; Oshida, K. Early dietary treatments with Lorenzo’s oil and docosahexaenoic acid for neurological development in a case with Zellweger syndrome. Brain Dev. 2007, 29, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Eliton, C.; Brucec, D.; Kennedyh, E. A comparison of the lipid and fatty acid profiles from the kernels of the fruit (nuts) of Ximenia caffra and Ricinodendron rautanenii from Zimbabwe. Ind. Crops Prod. 2008, 27, 29–32. [Google Scholar] [CrossRef]
- Alkasir, R.; Li, J.; Li, X.; Jin, M.; Zhu, B. Human gut micro-biota: The links with dementia development. Protein Cell 2016, 8, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Bagur, M.; Murcia, M.; Jiménez-Monreal, A.; Tur, J.; Bibiloni, M.; Alonso, G.; Martínez-Tomé, M. Influence of diet in multiple sclerosis:a systematic review. Adv. Nutr. 2017, 8, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, G.; Hadgkiss, E.; Weiland, T.; Pereira, N.; Marck, C.; van der Meer, D. Association offish consumption andomega-3 supplementation with quality of life, disabilityand disease activity in an international cohort of peoplewith multiple sclerosis. Int. J. Neurosci. 2013, 123, 792–801. [Google Scholar] [CrossRef]
- Ramirez-Ramirez, V.; Macias-Islas, M.; Ortiz, G.; Pacheco-Moises, F.; Torres-Sanchez, E.; Sorto-Gomez, T.; Cruz-Ramos, J.A.; Orozco-Aviña, G.; Celis De La Rosa, A.J. Efficacy offish oil on serum of TNFα, IL-1β, and IL-6 oxidative stress markers in multiple sclerosis treatedwith interferon beta-1b. Oxid. Med. Cell Longev. 2013, 2013, 709493. [Google Scholar] [CrossRef] [PubMed]
- Freitas, H.R.; Isaac, A.R.; Malcher-Lopes, R.; Diaz, B.L.; Trevenzoli, I.H.; De Melo Reis, R.A. Polyunsaturated fatty acids and endocannabinoids in health and disease. Nutr. Neurosci. 2018, 21, 695–714. [Google Scholar] [CrossRef] [PubMed]
- Stańdo, M.; Piątek, P.; Namiecinska, M.; Lewkowicz, P.; Lewkowicz, N. Omega-3 Polyunsaturated Fatty Acids EPA and DHA as an Adjunct to Non-Surgical Treatment of Periodontitis: A Randomized Clinical Trial. Nutrients 2020, 12, 2614. [Google Scholar] [CrossRef]
- Calon, F.; Cole, G. Neuroprotective action of omega-3 polyunsaturated fatty acids against neurodegenerative diseases: Evidence from animal studies. Prostaglandins Leukot. Essent. Fatty Acids. 2007, 77, 287–293. [Google Scholar] [CrossRef]

| NA Synthesis Substrates | Sources |
|---|---|
| Palmitic acid (16:0) | Palm oil, meat, poultry, olive oil, dairy products, soyabean oil, eggs, fish oil |
| Stearic acid (18:0) | Meat (beef), poultry, soyabean oil, olive oil, butter, sunflower oil, fish oil |
| Oleic acid (18:1 n-9) | Olive oil, soyabean oil, nuts, sesame oil, sunflower oil, fish oil |
| Eicosenoic acid (20:1 n-9) | Nuts, fish, plant oils, fish oil |
| Clinical Trial | Treatment Group | Placebo Group | Duration (Months) | Purpose | Clinical Trial ID | |
|---|---|---|---|---|---|---|
| Omega 3/omega 6 | Lipoic Acid and Omega-3 Fatty Acids for Alzheimer’s Disease | 34 + lipoic acid and fish oil concentrate | 33 + placebo lipoic acid plus placebo oil | 18 | To see if taking lipoic acid plus omega-3 fatty acids (omega-3s) can slow the Alzheimer’s disease (AD) process. | NCT01058941 |
| DHA (Docosahexaenoic Acid), an Omega 3 Fatty Acid, in Slowing the Progression of Alzheimer’s Disease (DHA) | 238 + DHA | 164 + placebo | 18 | To determine whether chronic DHA (docosahexaenoic acid) supplementation slows the progression of cognitive and functional decline in mild to moderate Alzheimer’s disease (AD). | NCT00440050 | |
| Fish Oil and Alpha Lipoic Acid in Treating Alzheimer’s Disease | 13 + fish oil concentrate | 13 + placebo oil (soybean oil) | 12 | To evaluate the effect of fish oil and alpha lipoic acid on inflammation, lipid levels, and oxidative stress. | NCT00090402 | |
| Fish Oil for the Treatment of Depression in Patients with Multiple Sclerosis | 21 + ish oil concentrate | 18 + soybean placebo | 3 | To determine whether fish oil can reduce depression in people with multiple sclerosis (MS). | NCT00122954 | |
| Efficacy of Fish Oil in Multiple Sclerosis (EFOMS) | 50 (RR-MS) + fish oil | No data | 12 | To evaluate the efficacy of fish oil supplementation on serum proinflammatory cytokines levels, oxidative stress markers, and disease progression in MS. | NCT01842191 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namiecinska, M.; Piatek, P.; Lewkowicz, P. Nervonic Acid Synthesis Substrates as Essential Components in Profiled Lipid Supplementation for More Effective Central Nervous System Regeneration. Int. J. Mol. Sci. 2024, 25, 3792. https://doi.org/10.3390/ijms25073792
Namiecinska M, Piatek P, Lewkowicz P. Nervonic Acid Synthesis Substrates as Essential Components in Profiled Lipid Supplementation for More Effective Central Nervous System Regeneration. International Journal of Molecular Sciences. 2024; 25(7):3792. https://doi.org/10.3390/ijms25073792
Chicago/Turabian StyleNamiecinska, Magdalena, Paweł Piatek, and Przemysław Lewkowicz. 2024. "Nervonic Acid Synthesis Substrates as Essential Components in Profiled Lipid Supplementation for More Effective Central Nervous System Regeneration" International Journal of Molecular Sciences 25, no. 7: 3792. https://doi.org/10.3390/ijms25073792
APA StyleNamiecinska, M., Piatek, P., & Lewkowicz, P. (2024). Nervonic Acid Synthesis Substrates as Essential Components in Profiled Lipid Supplementation for More Effective Central Nervous System Regeneration. International Journal of Molecular Sciences, 25(7), 3792. https://doi.org/10.3390/ijms25073792