Nerve Growth Factor Shows Biphasic Expression during Adjuvant-Induced Neurogenic Inflammation
Abstract
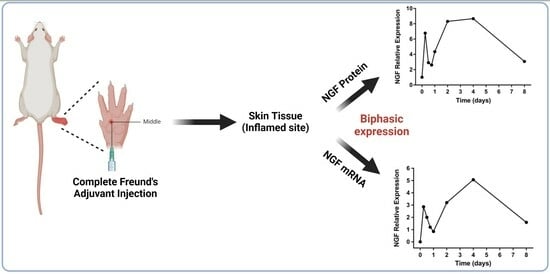
:1. Introduction
2. Results
2.1. Changes in Hind Paw Edema and Body Weight
2.2. Change in NGF-Immunoreactivity (-ir) during Inflammation
2.3. NGF Expression Shows Biphasic Response during Peripheral Inflammation
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Induction of Adjuvant-Induced Arthritis (AIA)
4.3. Hind Paw Edema and Body Weight
4.4. Thermolysin Treatment
4.5. Immunohistochemistry (IHC)
4.6. Quantitative Image Analysis
4.7. RNA Isolation and Quantitative Real-Time PCR
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denk, F.; Bennett, D.L.; McMahon, S.B. Nerve Growth Factor and Pain Mechanisms. Annu. Rev. Neurosci. 2017, 40, 307–325. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J.; Safieh-Garabedian, B.; Ma, Q.P.; Crilly, P.; Winter, J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience 1994, 62, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Ganju, P.; O’Bryan, J.P.; Der, C.; Winter, J.; James, I.F. Differential regulation of SHC proteins by nerve growth factor in sensory neurons and PC12 cells. Eur. J. Neurosci. 1998, 10, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Mizumura, K.; Murase, S. Role of nerve growth factor in pain. Handb. Exp. Pharmacol. 2015, 227, 57–77. [Google Scholar] [PubMed]
- Verma, V.; Sheikh, Z.; Ahmed, A.S. Nociception and role of immune system in pain. Acta Neurol. Belg. 2015, 115, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P.; Maggi, C.A. Dissociation of dorsal root ganglion neurons into afferent and efferent-like neurons. Neuroscience 1998, 86, 389–398. [Google Scholar] [PubMed]
- Chiu, I.M.; von Hehn, C.A.; Woolf, C.J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 2012, 15, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.J.; Allen, S.J.; Dawbarn, D. Targeting nerve growth factor in pain: What is the therapeutic potential? BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2008, 22, 349–359. [Google Scholar] [CrossRef]
- Miller, K.E.; Hoffman, E.M.; Sutharshan, M.; Schechter, R. Glutamate pharmacology and metabolism in peripheral primary afferents: Physiological and pathophysiological mechanisms. Pharmacol. Ther. 2011, 130, 283–309. [Google Scholar] [CrossRef]
- Harrington, A.W.; Ginty, D.D. Long-distance retrograde neurotrophic factor signalling in neurons. Nat. Rev. Neurosci. 2013, 14, 177–187. [Google Scholar] [CrossRef]
- Ye, M.; Lehigh, K.M.; Ginty, D.D. Multivesicular bodies mediate long-range retrograde NGF-TrkA signaling. eLife 2018, 7, e33012. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Guo, T.Z.; Li, W.W.; Hou, S.; Kingery, W.S.; Clark, J.D. Keratinocyte expression of inflammatory mediators plays a crucial role in substance P-induced acute and chronic pain. J. Neuroinflammation 2012, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.B.; Miller, K.E.; Schechter, R. Evaluation of rat epidermis and dermis following thermolysin separation: PGP 9.5 and Nav 1.8 localization. Soc. Neurosci. Abstr. 2010, 9, 584. [Google Scholar]
- Gujar, V.; Anderson, M.B.; Miller, K.E.; Pande, R.D.; Nawani, P.; Das, S. Separation of Rat Epidermis and Dermis with Thermolysin to Detect Site-Specific Inflammatory mRNA and Protein. J. Vis. Exp. 2021; 175, e59708. [Google Scholar] [CrossRef]
- Hoffman, E.M. The Role of Dorsal Root Ganglion Glutaminase in Nociception—A Novel Therapeutic Target for Inflammatory Pain. Ph.D. Thesis, Oklahoma State University, Ann Arbor, MI, USA, 2009. Volume 3356494. [Google Scholar]
- Zhang, Z. The Role of Dorsal Root Ganglion Glutaminase in Acute and Chronic Inflammatory Pain. Ph.D. Thesis, Oklahoma State University, Ann Arbor, MI, USA, 2013. Volume 3629945. Available online: http://argo.library.okstate.edu/login?url=https://search.proquest.com/docview/1562918358?accountid=4117 (accessed on 7 July 2019).
- Ji, R.R.; Xu, Z.Z.; Gao, Y.J. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Burma, N.E.; Leduc-Pessah, H.; Fan, C.Y.; Trang, T. Animal models of chronic pain: Advances and challenges for clinical translation. J. Neurosci. Res. 2017, 95, 1242–1256. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, R.; Jialal, I. Chronic Inflammation; StatPearls: Treasure Island, FL, USA, 2018. Available online: https://www.ncbi.nlm.nih.gov/pubmed/29630225 (accessed on 31 July 2019).
- Lamb, K.; Zhong, F.; Gebhart, G.F.; Bielefeldt, K. Experimental colitis in mice and sensitization of converging visceral and somatic afferent pathways. Am. J. Physiol. Gastrointest Liver Physiol. 2006, 290, G451–G457. [Google Scholar] [CrossRef] [PubMed]
- Gibbins, I.L.; Wattchow, D.; Coventry, B. Two immunohistochemically identified populations of calcitonin gene-related peptide (CGRP)-immunoreactive axons in human skin. Brain Res. 1987, 414, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Kemeny, L.; von Restorff, B.; Michel, G.; Ruzicka, T. Specific binding and lack of growth-promoting activity of substance P in cultured human keratinocytes. J. Invest. Dermatol. 1994, 103, 605–606. [Google Scholar] [CrossRef]
- Shi, X.; Wang, L.; Clark, J.D.; Kingery, W.S. Keratinocytes express cytokines and nerve growth factor in response to neuropeptide activation of the ERK1/2 and JNK MAPK transcription pathways. Regul. Pept. 2013, 186, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Viac, J.; Gueniche, A.; Doutremepuich, J.D.; Reichert, U.; Claudy, A.; Schmitt, D. Substance P and keratinocyte activation markers: An in vitro approach. Arch. Dermatol. Res. 1996, 288, 85–90. [Google Scholar] [CrossRef]
- McMahon, S.B.; Bennett, D.L.; Priestley, J.V.; Shelton, D.L. The biological effects of endogenous nerve growth factor on adult sensory neurons revealed by a trkA-IgG fusion molecule. Nat. Med. 1995, 1, 774–780. [Google Scholar] [CrossRef]
- Oddiah, D.; Anand, P.; McMahon, S.B.; Rattray, M. Rapid increase of NGF, BDNF and NT-3 mRNAs in inflamed bladder. Neuroreport 1998, 9, 1455–1458. [Google Scholar] [CrossRef] [PubMed]
- Leon, A.; Buriani, A.; Dal Toso, R.; Fabris, M.; Romanello, S.; Aloe, L.; Levi-Montalcini, R. Mast cells synthesize, store, and release nerve growth factor. Proc. Natl. Acad. Sci. USA 1994, 91, 3739–3743. [Google Scholar] [CrossRef] [PubMed]
- Heumann, R.; Lindholm, D.; Bandtlow, C.; Meyer, M.; Radeke, M.J.; Misko, T.P.; Shooter, E.; Thoenen, H. Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration, and regeneration: Role of macrophages. Proc. Natl. Acad. Sci. USA 1987, 84, 8735–8739. [Google Scholar] [CrossRef] [PubMed]
- Mantyh, P.W.; Koltzenburg, M.; Mendell, L.M.; Tive, L.; Shelton, D.L. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology 2011, 115, 189–204. [Google Scholar] [CrossRef]
- Dallos, A.; Kiss, M.; Polyanka, H.; Dobozy, A.; Kemeny, L.; Husz, S. Effects of the neuropeptides substance P, calcitonin gene-related peptide, vasoactive intestinal polypeptide and galanin on the production of nerve growth factor and inflammatory cytokines in cultured human keratinocytes. Neuropeptides 2006, 40, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, D.; Heumann, R.; Meyer, M.; Thoenen, H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature 1987, 330, 658–659. [Google Scholar] [CrossRef]
- Marz, P.; Heese, K.; Dimitriades-Schmutz, B.; Rose-John, S.; Otten, U. Role of interleukin-6 and soluble IL-6 receptor in region-specific induction of astrocytic differentiation and neurotrophin expression. Glia 1999, 26, 191–200. [Google Scholar] [CrossRef]
- Safieh-Garabedian, B.; Poole, S.; Allchorne, A.; Winter, J.; Woolf, C.J. Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br. J. Pharmacol. 1995, 115, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J.; Allchorne, A.; Safieh-Garabedian, B.; Poole, S. Cytokines, nerve growth factor and inflammatory hyperalgesia: The contribution of tumour necrosis factor alpha. Br. J. Pharmacol. 1997, 121, 417–424. [Google Scholar] [CrossRef]
- Edwards, R.H.; Selby, M.J.; Garcia, P.D.; Rutter, W.J. Processing of the native nerve growth factor precursor to form biologically active nerve growth factor. J. Biol. Chem. 1988, 263, 6810–6815. [Google Scholar] [CrossRef]
- Seidah, N.G.; Benjannet, S.; Pareek, S.; Savaria, D.; Hamelin, J.; Goulet, B.; Laliberte, J.; Lazure, C.; Chrétien, M.; Murphy, R.A. Cellular processing of the nerve growth factor precursor by the mammalian pro-protein convertases. Biochem. J. 1996, 314 Pt 3, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Mesentier-Louro, L.A.; De Nicolo, S.; Rosso, P.; De Vitis, L.A.; Castoldi, V.; Leocani, L.; Tirassa, P.; Rama, P.; Lambiase, A. Time-Dependent Nerve Growth Factor Signaling Changes in the Rat Retina During Optic Nerve Crush-Induced Degeneration of Retinal Ganglion Cells. Int. J. Mol. Sci. 2017, 18, 98. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shen, Y. An old method facing a new challenge: Re-visiting housekeeping proteins as internal reference control for neuroscience research. Life Sci. 2013, 92, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Turabelidze, A.; Guo, S.; DiPietro, L.A. Importance of housekeeping gene selection for accurate reverse transcription-quantitative polymerase chain reaction in a wound healing model. Wound Repair Regen. 2010, 18, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Gujar, V. Understanding the Mechanism of Nerve Growth Factor Signaling during Peripheral Inflammation. Ph.D. Thesis, Oklahoma State University, Stillwater, OK, USA, 2020. Available online: https://www.proquest.com/dissertations-theses/understanding-mechanism-nerve-growth-factor/docview/2572613459/se-2?accountid=45198 (accessed on 7 July 2019).
- Hoffman, E.M.; Schechter, R.; Miller, K.E. Fixative composition alters distributions of immunoreactivity for glutaminase and two markers of nociceptive neurons, Nav1.8 and TRPV1, in the rat dorsal root ganglion. J. Histochem. Cytochem. 2010, 58, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.M.; Zhang, Z.; Anderson, M.B.; Schechter, R.; Miller, K.E. Potential mechanisms for hypoalgesia induced by anti-nerve growth factor immunoglobulin are identified using autoimmune nerve growth factor deprivation. Neuroscience 2011, 193, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Bezuidenhout, J.A.; Pretorius, E. The Central Role of Acute Phase Proteins in Rheumatoid Arthritis: Involvement in Disease Autoimmunity, Inflammatory Responses, and the Heightened Risk of Cardiovascular Disease. Semin. Thromb Hemost. 2020, 46, 465–483. [Google Scholar] [CrossRef]
- Conti, F.; Ceccarelli, F.; Perricone, C.; Miranda, F.; Truglia, S.; Massaro, L.; Pacucci, V.A.; Conti, V.; Bartosiewicz, I.; Spinelli, F.R.; et al. Flare, persistently active disease, and serologically active clinically quiescent disease in systemic lupus erythematosus: A 2-year follow-up study. PLoS ONE 2012, 7, e45934. [Google Scholar] [CrossRef]
- Helmchen, B.; Weckauf, H.; Ehemann, V.; Wittmann, I.; Meyer-Scholten, C.; Berger, I. Expression pattern of cell cycle-related gene products in synovial stroma and synovial lining in active and quiescent stages of rheumatoid arthritis. Histol. Histopathol. 2005, 20, 365–372. [Google Scholar]
- Wenink, M.H.; Santegoets, K.C.; Roelofs, M.F.; Huijbens, R.; Koenen, H.J.; van Beek, R.; Joosten, I.; Meyer-Wentrup, F.; Mathsson, L.; Ronnelid, J.; et al. The inhibitory Fc gamma IIb receptor dampens TLR4-mediated immune responses and is selectively upregulated on dendritic cells from rheumatoid arthritis patients with quiescent disease. J. Immunol. 2009, 183, 4509–4520. [Google Scholar] [CrossRef]




| Gene | Primer Sequence |
|---|---|
| Nerve growth factor (NGF) | NGF-F: 5′-GTGGACCCCAAACTGTTTAAGAAACGG-3′ NGF-R: 5′-GTGAGTCCTGTTGAAGGAGATTGTACCATG-3’ |
| GAPDH | GAPDH-F: 5′- GAACCACGAGAAATATGACAACTCCCTCAAG-3′ GAPDH-R: 5′- GCAGTGATGGCATGGACTGTGG-3′ |
| Primary Antibodies | Dilutions | Secondary Antibodies | Dilutions | |
|---|---|---|---|---|
| Immunohistochemistry | NGF Anti-mouse (E-12, Santa Cruz, Dallas, TX, USA) | 1:1000 | Donkey anti-mouse Alexa Flour 555 (Invitrogen; Carlsbad, CA, USA) | 1:1000 |
| PGP 9.5 Anti-rabbit (Cederlane Labs, Burlington, ON, Canada) | 1:10,000 | Donkey anti-rabbit FITC 488 (Invitrogen; Carlsbad, CA, USA) | 1:1000 | |
| Western Blot | NGF Anti-mouse (E-12, Santa Cruz, Dallas, TX, USA) | 1:1000 | Goat anti-mouse IgG (Promega; Madison, WI, USA) | 1:1000 |
| GAPDH (Santa Cruz, Dallas TX, USA) | 1:1000 | Goat anti-rabbit IgG (Promega; Madison, WI, USA) | 1:1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gujar, V.; Pande, R.D.; Das, S. Nerve Growth Factor Shows Biphasic Expression during Adjuvant-Induced Neurogenic Inflammation. Int. J. Mol. Sci. 2024, 25, 4029. https://doi.org/10.3390/ijms25074029
Gujar V, Pande RD, Das S. Nerve Growth Factor Shows Biphasic Expression during Adjuvant-Induced Neurogenic Inflammation. International Journal of Molecular Sciences. 2024; 25(7):4029. https://doi.org/10.3390/ijms25074029
Chicago/Turabian StyleGujar, Vikramsingh, Radhika D. Pande, and Subhas Das. 2024. "Nerve Growth Factor Shows Biphasic Expression during Adjuvant-Induced Neurogenic Inflammation" International Journal of Molecular Sciences 25, no. 7: 4029. https://doi.org/10.3390/ijms25074029
APA StyleGujar, V., Pande, R. D., & Das, S. (2024). Nerve Growth Factor Shows Biphasic Expression during Adjuvant-Induced Neurogenic Inflammation. International Journal of Molecular Sciences, 25(7), 4029. https://doi.org/10.3390/ijms25074029