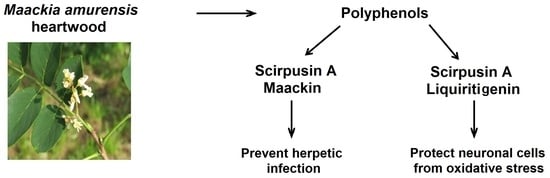
Polyphenols from Maackia amurensis Heartwood Protect Neuronal Cells from Oxidative Stress and Prevent Herpetic Infection
Abstract
:1. Introduction
2. Results
2.1. Polyphenolic Compounds from M. amurensis Heartwood
2.2. Determination of Absolute Configurations for Maackiazin (10) and Scirpusin A (13)
2.3. In Vitro Model of 6-OHDA Induced Neurotoxicity (Cell Viability Test)
2.4. Reactive Oxygen Species (ROS) Analysis in 6-OHDA-Treated Cells
2.5. Mitochondrial Membrane Potential (MMP) Detection
2.6. Cell Viability and Reactive Oxygen Species (ROS) Analysis in PQ-Treated Cells
2.7. Apoptosis
2.8. Virucidal Activity of Polyphenolic Compounds from M. amurensis (CPE Inhibition Assay)
2.9. Virucidal Activity of Polyphenolic Compounds from M. amurensis (RT-PCR Assay)
2.10. The Effect of Polyphenols on the Early Stages of HSV-1 Infection
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction and Isolation
4.3. General Experimental Procedures
4.4. HPLC–PDA–MS
4.5. Neuro-2a Cell Line and Culture Condition
4.6. In Vitro Model of 6-OHDA and PQ-Induced Neurotoxicity (Cell Viability Test)
4.7. Reactive Oxygen Species (ROS) Analysis in 6-OHDA- and PQ-Treated Cells
4.8. Mitochondrial Membrane Potential (MMP) Detection
4.9. Apoptosis
4.10. HSV-1 Virus and Vero Cell Culture
4.11. Cytotoxicity of the Tested Compounds against Vero Cells
4.12. Virucidal Activity of Polyphenolic Compounds from M. amurensis
4.12.1. Cytopathic Effect (CPE) Inhibition Assay
4.12.2. Real-Time Polymerase Chain Reaction (RT-PCR) Assay
4.13. The Effect of Polyphenols on the Early Stages of HSV-1 Infection
4.13.1. Pretreatment of Cells with Polyphenolic Compounds
4.13.2. Treatment of Virus-Infected Cells with Polyphenols
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fedoreev, S.A.; Kulesh, N.I.; Glebko, L.I.; Pokushalova, T.V.; Veselova, M.V.; Saratikov, A.S.; Vengerovskii, A.I.; Chuchalin, V.S. Maksar: A preparation based on Amur Maackia. Pharm. Chem. J. 2004, 38, 605–610. [Google Scholar] [CrossRef]
- Kulesh, N.I.; Fedoreev, S.A.; Veselova, M.V.; Denisenko, V.A.; Grigorchuk, V.P. Isoflavone glycosides from root bark of Maackia amurensis. Chem. Nat. Compd. 2020, 56, 415–419. [Google Scholar] [CrossRef]
- Veselova, M.V.; Fedoreyev, S.A.; Tarbeeva, D.V.; Kulesh, N.I.; Kalinovskiy, A.I.; Kuzmich, A.S.; Kim, N.Y.; Grigorchuk, V.P. Cytotoxic prenylated polyphenolic compounds from Maackia amurensis root bark. Nat. Prod. Commun. 2017, 12, 1037–1040. [Google Scholar] [CrossRef]
- Plotnikova, A.M.; Shulgau, Z.T.; Plotnikova, T.M.; Aliev, O.I.; Kulesh, N.I.; Mischenko, N.P.; Fedoreyev, S.A. Antithrombogenic and Antiplatelet activities of extract from Maackia amurensis wood. Bull. Exp. Biol. Med. 2009, 2, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Fedoreev, S.A.; Kulesh, N.I.; Mishchenko, N.P.; Ermakova, S.P.; Zvjagintseva, T.N. Anticancer Drug. RU Patent 2414920 C1, 1 December 2009. [Google Scholar]
- Utkina, N.K.; Kulesh, N.I. Antioxidant activity of polyphenols and polyphenol complex from the far-eastern tree Maackia amurensis. Pharm. Chem. J. 2012, 46, 488–491. [Google Scholar] [CrossRef]
- Putilova, E.A.; Ivanis, V.A.; Skljar, L.F. Clinical and immunological effects Maksar in chronic viral hepatitis. Fundam. Res. 2011, 9, 484–487. [Google Scholar]
- Krylova, N.V.; Iunikhina, O.V.; Fedoreev, S.A.; Pott, A.B.; Persiyanova, E.V.; Mishchenko, N.P.; Tarbeeva, D.V.; Shchelkanov, M.Y. Anti-SARS-CoV-2 Activity of a Polyphenolic Complex from Maackia amurensis. Bull. Exp. Biol. Med. 2023, 176, 187–190. [Google Scholar] [CrossRef]
- Cetin, A. In silico studies on stilbenolignan analogues as SARS-CoV-2 Mpro inhibitors. Chem. Phys. Lett. 2021, 771, 138563. [Google Scholar] [CrossRef]
- Elsbaey, M.; Ibrahim, M.A.A.; Bar, F.A.; Elgazar, A.A. Chemical constituents from coconut waste and their in silico evaluation as potential antiviral agents against SARS-CoV-2. S. Afr. J. Bot. 2021, 141, 278–289. [Google Scholar] [CrossRef]
- Krylova, N.V.; Fedoreev, S.A.; Iunikhina, O.V.; Mishchenko, N.P.; Pott, A.B.; Tarbeeva, D.V.; Shchelkanov, M.Y. Antiviral Agent against Human Herpesvirus Type 1 and Enterovirus B. RU Patent 2798659 C1, 23 June 2023. [Google Scholar]
- He, Q.; Liu, H.; Huang, C.; Wang, R.; Luo, M.; Lu, W. Herpes Simplex Virus 1-Induced Blood-Brain Barrier Damage Involves Apoptosis Associated with GM130-Mediated Golgi Stress. Front. Mol. Neurosci. 2020, 13, 2. [Google Scholar] [CrossRef]
- Santana, S.; Sastre, I.; Recuero, M.; Bullido, M.J.; Aldudo, J. Oxidative stress enhance neurodegeneration markers induced by herpes simplex virus type 1 infection in human neuroblastoma cells. PLoS ONE 2013, 8, e75842. [Google Scholar] [CrossRef] [PubMed]
- Kavouras, J.H.; Prandovszky, E.; Valyi-Nagy, K.; Kovacs, S.K.; Tiwari, V.; Kovacs, M.; Shukla, D.; Valyi-Nagy, T. Herpes simplex virus type 1 infection induces oxidative stress and the release of bioactive lipid peroxidation by-products in mouse P19 neural cell cultures. J. Neurovirol. 2007, 13, 416–425. [Google Scholar] [CrossRef] [PubMed]
- De Chiara, G.; Marcocci, M.E.; Sgarbanti, R.; Civitelli, L.; Ripoli, C.; Piacentini, R.; Garaci, E.; Grassi, C.; Palamara, A.T. Infectious agents and neurodegeneration. Mol. Neurobiol. 2012, 46, 614–638. [Google Scholar] [CrossRef] [PubMed]
- Berry, N.; Suspène, R.; Caval, V.; Khalfi, P.; Beauclair, G.; Rigaud, S.; Blanc, H.; Vignuzzi, M.; Wain-Hobson, S.; Vartanian, J.P. Herpes simplex virus type 1 infection disturbs the mitochondrial network, leading to type I interferon production through the RNA Polymerase III/RIG-I pathway. mBio 2021, 12, e0255721. [Google Scholar] [CrossRef] [PubMed]
- Manivanh, R.; Mehrbach, J.; Charron, A.J.; Grassetti, A.; Cerón, S.; Taylor, S.A.; Cabrera, J.R.; Gerber, S.; Leib, D.A. Herpes simplex virus 1 ICP34.5 alters mitochondrial dynamics in neurons. J. Virol. 2020, 94, e01784-19. [Google Scholar] [CrossRef] [PubMed]
- Limongi, D.; Baldelli, S. Redox imbalance and viral infections in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2016, 2016, 6547248. [Google Scholar] [CrossRef]
- Cymerys, J.; Chodkowski, M.; Slonska, A.; Krzyzowska, M.; Banbura, M.W. Disturbances of mitochondrial dynamics in cultured neurons infected with human herpesvirus type 1 and type 2. J. Neurovirol. 2019, 25, 765–782. [Google Scholar] [CrossRef] [PubMed]
- Area-Gomez, E.; de Groof, A.; Bonilla, E.; Montesinos, J.; Tanji, K.; Boldogh, I.; Pon, L.; Schon, E.A. A key role for MAM in mediating mitochondrial dysfunction in Alzheimer disease. Cell Death Dis. 2018, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, A.; Kumar, K.R.; Sue, C.M. New insights into the complex role of mitochondria in Parkinson’s disease. Prog. Neurobiol. 2019, 177, 73–93. [Google Scholar] [CrossRef]
- Tarbeeva, D.V.; Berdyshev, D.V.; Pislyagin, E.A.; Menchinskaya, E.S.; Kim, N.Y.; Kalinovskiy, A.I.; Krylova, N.V.; Iunikhina, O.V.; Persiyanova, E.V.; Shchelkanov, M.Y.; et al. Neuroprotective and antiherpetic properties of polyphenolic compounds from Maackia amurensis heartwood. Molecules 2023, 28, 2593. [Google Scholar] [CrossRef]
- Levai, A. Utilization of the chiroptical spectroscopies for the structure elucidation of flavonoids and related benzopyran derivatives. Acta Chim. Slov. 1998, 45, 267–284. [Google Scholar] [CrossRef]
- Moreno-Celis, U.; López-Martínez, F.J.; Cervantes-Jiménez, R.; Ferríz-Martínez, R.A.; Blanco-Labra, A.; García-Gasca, T. Tepary Bean (Phaseolus acutifolius) Lectins Induce Apoptosis and Cell Arrest in G0/G1 by P53 (Ser46) Phosphorylation in Colon Cancer Cells. Molecules 2020, 25, 1021. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, G.G.; Coronel, O.A.; Trabuco, A.C.; Bazán, D.E.; Russo, R.R.; Alvarenga, N.L.; Aquino, V.H. Steroidal saponins from the roots of Solanum sisymbriifolium Lam. (Solanaceae) have inhibitory activity against dengue virus and yellow fever virus. Braz. J. Med. Biol. Res. 2021, 54, e10240. [Google Scholar] [CrossRef]
- Low, Z.X.; OuYong, B.M.; Hassandarvish, P.; Poh, C.L.; Ramanathan, B. Antiviral activity of silymarin and baicalein against dengue virus. Sci. Rep. 2021, 11, 21221. [Google Scholar] [CrossRef]
- Fernandes, L.S.; da Silva, M.L.; Dias, R.S.; da S. Lucindo, M.S.; da Silva, Í.E.P.; Silva, C.C.; Teixeira, R.R.; de Paula, S.O. Evaluation of Antiviral Activity of Cyclic Ketones against Mayaro Virus. Viruses 2021, 13, 2123. [Google Scholar] [CrossRef]
- Bove, J.; Prou, D.; Perier, C.; Przedborski, S. Toxin-induced models of Parkinson’s disease. NeuroRX 2005, 2, 484–494. [Google Scholar] [CrossRef]
- Bastias-Candia, S.; Zolezzi, J.M.; Inestrosa, N.C. Revisiting the paraquat-induced sporadic Parkinson’s disease-like model. Mol. Neurobiol. 2019, 56, 1044–1055. [Google Scholar] [CrossRef]
- Okawara, M.; Katsuki, H.; Kurimoto, E.; Shibata, H.; Kume, T.; Akaike, A. Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochem. Pharmacol. 2007, 73, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Sebastiano, M.; Chastel, O.; de Thoisy, B.; Eens, M.; Costantini, D. Oxidative stress favours herpes virus infection in vertebrates: A meta-analysis. Curr. Zool. 2016, 62, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Ngan, L.T.M.; Jang, M.J.; Kwon, M.J.; Ahn, Y.J. Antiviral activity and possible mechanism of action of constituents identified in Paeonia lactiflora root toward human rhinoviruses. PLoS ONE 2015, 10, e0121629. [Google Scholar] [CrossRef]
- Weislow, O.S.; Kiser, R.; Fine, D.L.; Bader, J.; Shoemaker, R.H.; Boyd, M.R. New soluble-formazan assay for HIV-1 cytopathic effects: Application to high-flux screening of synthetic and natural products for AIDS-antiviral activity. J. Natl. Cancer Inst. 1989, 81, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Shchelkanov, M.Y.; Sakhuriya, I.B.; Burunova, V.V.; Pavlova, T.V.; Kornilaeva, G.V.; Karamov, E.V.; Eremin, V.F. Dehydrogenase activity of infected cells and biological properties of various HIV-1 variants. Biochemistry 1999, 64, 513–519. [Google Scholar]
- Bovard, D.; van der Toorn, M.; Schlage, W.K.; Constant, S.; Renggli, K.; Peitsch, M.C.; Hoeng, J. Iota-carrageenan extracted from red algae is a potent inhibitor of SARS-CoV-2 infection in reconstituted human airway epithelia. Biochem. Biophys. Rep. 2022, 29, 101187. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Resveratrol as a novel anti-herpes simplex virus nutraceutical agent: An overview. Viruses 2018, 10, 473. [Google Scholar] [CrossRef]
- Mattio, L.M.; Catinella, G.; Pinto, A.; Dallavalle, S. Natural and nature-inspired stilbenoids as antiviral agents. Eur. J. Med. Chem. 2020, 202, 112541. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]









| Live | Early apop. | Late apop./Dead | Dead | |
|---|---|---|---|---|
| Control | 90.53 ± 0.68 | 4.59 ± 0.32 | 4.3 ± 0.29 | 0.63 ± 0.05 |
| 6-OHDA | 44.14 ± 1.34 | 42.62 ± 2.3 | 12.15 ± 1.34 | 1.11 ± 0.37 |
| 3 | 67.89 ± 1.52 * | 19.63 ± 0.79 * | 11.31 ± 0.49 | 1.18 ± 0.24 |
| 6 | 61.83 ± 0.09 * | 27.67 ± 1.09 * | 10.89 ± 0.86 | 0.61 ± 0.13 |
| 13 | 56.90 ± 1.32 * | 30.18 ± 1.13 * | 12.2 ± 0.34 | 0.73 ± 0.15 |
| Compounds | CC50 | IC50 | SI | ||
|---|---|---|---|---|---|
| µg/mL | µM | µg/mL | µM | ||
| 1 | 123.0 ± 14.8 | 539.5 ± 64.9 | 9.6 ± 1.4 | 42.1 ± 6.1 | 12.8 ± 1.9 |
| 2 | 133.8 ± 20.1 | 495.6 ± 74.4 | 16.7 ± 2.3 | 61.9 ± 8.5 | 8.0 ± 1.0 |
| 4 | 152.1 ± 22.8 | 535.6 ± 80.3 | 28.3 ± 3.7 | 99.6 ± 13.0 | 5.4 ± 0.7 |
| 7 | 155.0 ± 23.2 | 574.1 ± 85.9 | 74.5 ± 11.1 | 275.9 ± 41.1 | 2.1 ± 0.3 |
| 9 | 269.6 ± 35.0 | 554.7 ± 72.0 | 2.4 ± 0.3 | 4.9 ± 0.6 | 112.3 ± 13.5 |
| 10 | 156.4 ± 18.8 | 297.3 ± 35.7 | 6.6 ± 0.9 | 12.5 ± 1.7 | 23.7 ± 3.1 |
| 11 | 250.2 ± 30.0 | 1025.4 ± 123.0 | 6.7 ± 0.9 | 27.4 ± 3.7 | 37.3 ± 5.2 |
| 13 | 199.4 ± 23.9 | 424.3 ± 50.1 | 2.6 ± 0.3 | 5.5 ± 0.6 | 76.7 ± 9.9 |
| 14 | 162.4 ± 17.9 | 316.0 ± 34.8 | 8.6 ± 1.2 | 16.7 ± 2.3 | 18.9 ± 2.8 |
| Acyclovir | >1000 | NA * | NA * | NA * | NA * |
| Compounds | Concentration | Ct | −ΔCt | 2−ΔCt | log10 | |
|---|---|---|---|---|---|---|
| µg/mL | µM | |||||
| 1 | 10 | 24.6 ± 2.9 * | −6.5 ± 0.8 | 0.0110 ± 0.0014 | −1.9 ± 0.2 * | |
| 1 | 20.4 ± 2.8 | −2.3 ± 0.2 | 0.203 ± 0.024 | −0.7 ± 0.09 | ||
| 0.1 | 18.1 ± 2.2 | 0 | 1.0 | 0 | ||
| 2 | 10 | 22.4 ± 2.4 * | −4.3 ± 0.5 | 0.0508 ± 0.0061 | −1.3 ± 0.2 * | |
| 1 | 18.4 ± 2.4 | −0.30 ± 0.03 | 0.812 ± 0.110 | −0.09 ± 0.01 | ||
| 0.1 | 18.1 ± 2.2 | 0 | 1.0 | 0 | ||
| 4 | 10 | 20.7 ± 2.3 | −2.6 ± 0.3 | 0.165 ± 0.021 | −0.8 ± 0.1 | |
| 1 | 19.0 ± 2.1 | −0.9 ± 0.1 | 0.536 ± 0.064 | −0.30 ± 0.04 | ||
| 0.1 | 18.1 ± 2.2 | 0 | 1.0 | 0 | ||
| 7 | 10 | 19.8 ± 2.4 | −1.75 ± 0.20 | 0.297 ± 0.04 | −0.50 ± 0.06 | |
| 1 | 18.3 ± 2.2 | −0.20 ± 0.02 | 0.870 ± 0.11 | −0.060 ± 0.008 | ||
| 0.1 | 18.1 ± 2.2 | 0 | 1.0 | 0 | ||
| 9 | 10 | 33.3 ± 4.3 * | −15.2 ± 1.8 | 0.0000266 ± 0.000003 | −4.6 ± 0.6 * | |
| 1 | 25.9 ± 2.8 * | −7.8 ± 0.9 | 0.00449 ± 0.0006 | −2.3 ± 0.3 * | ||
| 0.1 | 18.6 ± 2.4 | −0.50 ± 0.05 | 0.707 ± 0.092 | −0.15 ± 0.02 | ||
| 10 | 10 | 28.0 ± 3.9 * | −9.9 ± 1.1 | 0.00105 ± 0.0001 | −2.9 ± 0.4 * | |
| 1 | 20.2 ± 2.6 | −2.1 ± 0.3 | 0.233 ± 0.03 | −0.6 ± 0.08 | ||
| 0.1 | 18.4 ± 2.4 | −0.300 ± 0.03 | 0.812 ± 0.11 | −0.09 ± 0.01 | ||
| 11 | 10 | 30.2 ± 3.9 * | −12.1 ± 1.4 | 0.000228 ± 0.0003 | −3.6 ± 0.5 * | |
| 1 | 24.5 ± 3.4 * | −6.4 ± 0.8 | 0.0118 ± 0.0015 | −1.9 ± 0.2 * | ||
| 0.1 | 18.4 ± 2.4 | −0.30 ± 0.03 | 0.812 ± 0.11 | −0.09 ± 0.01 | ||
| 13 | 10 | 31.2 ± 3.7 * | −13.1 ± 1.6 | 0.000114 ± 0.00001 | −3.9 ± 0.5 * | |
| 1 | 25.8 ± 3.3 * | −7.7 ± 0.8 | 0.00481 ± 0.0006 | −2.3 ± 0.3 * | ||
| 0.1 | 18.6 ± 2.4 | −0.50 ± 0.05 | 0.707 ± 0.092 | −0.15 ± 0.02 | ||
| 14 | 10 | 27.1 ± 3.8 * | −9.0 ± 1.2 | 0.00195 ± 0.00020 | −2.7 ± 0.3 * | |
| 1 | 19.2 ± 2.5 | −1.1 ± 0.1 | 0.466 ± 0.056 | −0.33 ± 0.04 | ||
| 0.1 | 18.4 ± 2.4 | −0.30 ± 0.03 | 0.812 ± 0.110 | −0.09 ± 0.01 | ||
| Virus control (DMSO) | 18.1 ± 2.2 | 0 | 1.0 | 0 | ||
| Cell control (DMSO) | ≥37.0 | |||||
| Compounds | CC50 (µg/mL) | Pretreatment of Cells | Simultaneous Treatment * | Treatment of Infected Cells | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (µg/mL) | IC50 (µM/mL) | SI | IC50 (µg/mL) | IC50 (µM/mL) | SI | IC50 (µg/mL) | IC50 (µM/mL) | SI | ||
| 9 | 269.6 ± 35.0 | 70.3 ± 9.1 | 144.6 ± 18.7 | 3.8 ± 0.5 | 17.6 ± 2.3 | 36.2 ± 4.7 | 15.3 ± 1.9 | 24.5 ± 3.2 | 50.4 ± 6.6 | 11.0 ± 1.4 |
| 13 | 199.4 ± 23.9 | 47.2 ± 6.1 | 100.4 ± 13.0 | 4.2 ± 0.6 | 13.8 ± 1.6 | 29.4 ± 3.4 | 14.4 ± 1.7 | 20.2 ± 2.8 | 43.0 ± 6.0 | 9.8 ± 1.2 |
| Acyclovir | >1000 | NA | 2.1 ± 0.3 | 9.3 ± 1.3 | 430 | 0.4 ± 0.05 | 1.8 ± 0.2 | >2500 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarbeeva, D.V.; Pislyagin, E.A.; Menchinskaya, E.S.; Berdyshev, D.V.; Krylova, N.V.; Iunikhina, O.V.; Kalinovskiy, A.I.; Shchelkanov, M.Y.; Mishchenko, N.P.; Aminin, D.L.; et al. Polyphenols from Maackia amurensis Heartwood Protect Neuronal Cells from Oxidative Stress and Prevent Herpetic Infection. Int. J. Mol. Sci. 2024, 25, 4142. https://doi.org/10.3390/ijms25084142
Tarbeeva DV, Pislyagin EA, Menchinskaya ES, Berdyshev DV, Krylova NV, Iunikhina OV, Kalinovskiy AI, Shchelkanov MY, Mishchenko NP, Aminin DL, et al. Polyphenols from Maackia amurensis Heartwood Protect Neuronal Cells from Oxidative Stress and Prevent Herpetic Infection. International Journal of Molecular Sciences. 2024; 25(8):4142. https://doi.org/10.3390/ijms25084142
Chicago/Turabian StyleTarbeeva, Darya V., Evgeny A. Pislyagin, Ekaterina S. Menchinskaya, Dmitrii V. Berdyshev, Natalya V. Krylova, Olga V. Iunikhina, Anatoliy I. Kalinovskiy, Mikhail Y. Shchelkanov, Natalia P. Mishchenko, Dmitry L. Aminin, and et al. 2024. "Polyphenols from Maackia amurensis Heartwood Protect Neuronal Cells from Oxidative Stress and Prevent Herpetic Infection" International Journal of Molecular Sciences 25, no. 8: 4142. https://doi.org/10.3390/ijms25084142
APA StyleTarbeeva, D. V., Pislyagin, E. A., Menchinskaya, E. S., Berdyshev, D. V., Krylova, N. V., Iunikhina, O. V., Kalinovskiy, A. I., Shchelkanov, M. Y., Mishchenko, N. P., Aminin, D. L., & Fedoreyev, S. A. (2024). Polyphenols from Maackia amurensis Heartwood Protect Neuronal Cells from Oxidative Stress and Prevent Herpetic Infection. International Journal of Molecular Sciences, 25(8), 4142. https://doi.org/10.3390/ijms25084142