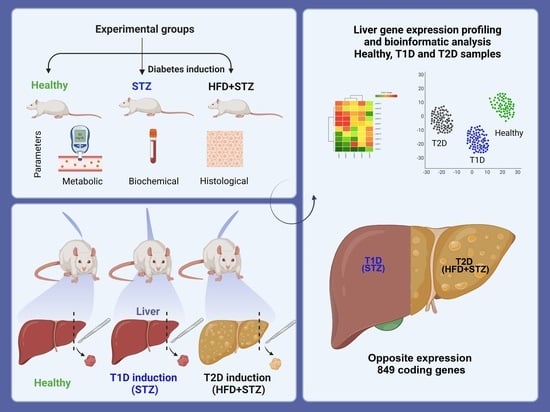
Comparative Screening of the Liver Gene Expression Profiles from Type 1 and Type 2 Diabetes Rat Models
Abstract
:1. Introduction
2. Results
2.1. STZ Administration Reduced the Body Weight Gain in the STZ Model
2.2. Hyperglycemia Is Maintained within a Narrower Value Range in the HFD + STZ Model Compared to the STZ Model
2.3. HFD + STZ and STZ Models Exhibit Impaired Glucose Tolerance but Insulin Resistance Is Only Found in the HDF + STZ Animals
2.4. Comparison of Biochemical Parameters in the Experimental Diabetes Models
2.5. Hepatomegaly and Liver Steatosis Were Only Developed in the HFD + STZ Model
2.6. Liver Steatosis Is Clearly Appreciated in the HFD + STZ Animals
2.7. The Pancreas of STZ Rats Contains a Reduced Number of Insulin-Positive Cells Compared to That of the HFD + STZ Model
2.8. Liver Gene Expression Profile by DNA Microarrays
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Diabetes Induction
4.2.1. Type 1 Diabetes Mellitus Induction (STZ Model)
4.2.2. Type 2 Diabetes Mellitus Induction (HFD + STZ Model)
4.2.3. Formulation of the High-Fat Diet (HFD)
4.2.4. Diabetes Confirmation after Chemical Induction
4.3. Oral Glucose Tolerance Test (OGTT)
4.4. Insulin Tolerance Test (ITT)
4.5. Blood and Tissue Collection
4.6. Biochemical Parameters
4.7. Liver Index
4.8. Histopathology Assessment
4.9. Insulin Immunohistochemistry (IHC)
4.10. RNA Extraction
4.11. DNA Microarray
4.12. Bioinformatic Analysis
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eizirik, D.L.; Pasquali, L.; Cnop, M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: Different pathways to failure. Nat. Rev. Endocrinol. 2020, 16, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Young, J.L.; Wang, K.; Qian, Y.; Cai, L. Diabetic-induced alterations in hepatic glucose and lipid metabolism: The role of type 1 and type 2 diabetes mellitus (Review). Mol. Med. Rep. 2020, 22, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Podell, B.K.; Ackart, D.F.; Richardson, M.A.; DiLisio, J.E.; Pulford, B.; Basaraba, R.J. A model of type 2 diabetes in the guinea pig using sequential diet-induced glucose intolerance and streptozotocin treatment. Dis. Model. Mech. 2017, 10, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Jeddi, S. Anti-obesity and anti-diabetic effects of nitrate and nitrite. Nitric Oxide 2017, 70, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar] [PubMed]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Correa, E.; González-Pérez, I.; Clavel-Pérez, P.I.; Contreras-Vargas, Y.; Carvajal, K. Biochemical and nutritional overview of diet-induced metabolic syndrome models in rats: What is the best choice? Nutr. Diabetes 2020, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Gheibi, S.; Kashfi, K.; Ghasemi, A. A practical guide for induction of type-2 diabetes in rat: Incorporating a high-fat diet and streptozotocin. Biomed. Pharmacother. 2017, 95, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Soto-Luna, I.C.; García-López, P.M.; Vargas-Guerrero, B.; Guzmán, T.J.; Domínguez-Rosales, J.A.; Gurrola-Díaz, C.M. Lupin protein isolate improves insulin sensitivity and steatohepatitis in vivo and modulates the expression of the Fasn, Gys2, and Gsk3b genes. Food Sci. Nutr. 2021, 9, 2549–2560. [Google Scholar] [CrossRef] [PubMed]
- Megyesi, C.; Samols, E.; Marks, V. Glucose tolerance and diabetes in chronic liver disease. Lancet 1967, 2, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E.; Targher, G. Increased risk of cardiovascular disease and chronic kidney disease in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Cusi, K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022, 10, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Combs, T.P.; Marliss, E.B. Adiponectin signaling in the liver. Rev. Endocr. Metab. Disord. 2014, 15, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lui, D.T.; Lam, K.S. Non-alcoholic fatty liver disease and type 2 diabetes: An update. J. Diabetes Investig. 2022, 13, 930–940. [Google Scholar] [CrossRef] [PubMed]
- Sadi, G.; Baloğlu, M.C.; Pektaş, M.B. Differential gene expression in liver tissues of streptozotocin-induced diabetic rats in response to resveratrol treatment. PLoS ONE 2015, 10, e0124968. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Gaikwad, A.B.; Tikoo, K. Hepatic expression profiling shows involvement of PKC epsilon, DGK eta, Tnfaip, and Rho kinase in type 2 diabetic nephropathy rats. J. Cell Biochem. 2010, 111, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, E.; Giampietro, O. Proposal open for discussion: Defining agreed diagnostic procedures in experimental diabetes research. J. Ethnopharmacol. 2008, 115, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.C.; Li, Y.; Chang, C.H.; Shieh, J.P.; Cheng, J.T.; Cheng, K.C. Investigation of insulin resistance in the popularly used four rat models of type-2 diabetes. Biomed. Pharmacother. 2018, 101, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.L.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Jin, Y.X.; Shen, W.; Neng, J.; Wu, T.; Li, Y.J.; Fu, Z.W. Low dose streptozotocin (STZ) combined with high energy intake can effectively induce type 2 diabetes through altering the related gene expression. Asia Pac. J. Clin. Nutr. 2007, 16 (Suppl. S1), 412–417. [Google Scholar]
- Skovsø, S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J. Diabetes Investig. 2014, 5, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, P.; Xu, Y.; Meng, X. Metabolomic Analysis of Biochemical Changes in the Plasma of High-Fat Diet and Streptozotocin-Induced Diabetic Rats after Treatment with Isoflavones Extract of Radix Puerariae. Evid. Based Complement. Altern. Med. 2016, 2016, 4701890. [Google Scholar] [CrossRef] [PubMed]
- Asrafuzzaman, M.; Cao, Y.; Afroz, R.; Kamato, D.; Gray, S.; Little, P.J. Animal models for assessing the impact of natural products on the aetiology and metabolic pathophysiology of Type 2 diabetes. Biomed. Pharmacother. 2017, 89, 1242–1251. [Google Scholar] [CrossRef]
- Premilovac, D.; Gasperini, R.J.; Sawyer, S.; West, A.; Keske, M.A.; Taylor, B.V.; Foa, L. A New Method for Targeted and Sustained Induction of Type 2 Diabetes in Rodents. Sci. Rep. 2017, 7, 14158. [Google Scholar] [CrossRef] [PubMed]
- Sampath, C.; Rashid, M.R.; Sang, S.; Ahmedna, M. Green tea epigallocatechin 3-gallate alleviates hyperglycemia and reduces advanced glycation end products via nrf2 pathway in mice with high fat diet-induced obesity. Biomed. Pharmacother. 2017, 87, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Rey, M.; Kruse, M.S.; Magrini-Huamán, R.N.; Coirini, H. High-Fat Diets and LXRs Expression in Rat Liver and Hypothalamus. Cell Mol. Neurobiol. 2019, 39, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Carbajo-Pescador, S.; Porras, D.; García-Mediavilla, M.V.; Martínez-Flórez, S.; Juarez-Fernández, M.; Cuevas, M.J.; Mauriz, J.L.; González-Gallego, J.; Nistal, E.; Sánchez-Campos, S. Beneficial effects of exercise on gut microbiota functionality and barrier integrity, and gut-liver crosstalk in an in vivo model of early obesity and non-alcoholic fatty liver disease. Dis. Model. Mech. 2019, 12, dmm039206. [Google Scholar] [CrossRef] [PubMed]
- Aboulmagd, Y.M.; El-Bahy, A.A.Z.; Menze, E.T.; Azab, S.S.; El-Demerdash, E. Role of linagliptin in preventing the pathological progression of hepatic fibrosis in high fat diet and streptozotocin-induced diabetic obese rats. Eur. J. Pharmacol. 2020, 881, 173224. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, K.A.A.; Ahmed, H.M.S.; Sharaf, H.A.; El-Nekeety, A.A.; Abdel-Aziem, S.H.; Mehaya, F.M.; Abdel-Wahhab, M.A. Encapsulation of cinnamon oil in whey protein counteracts the disturbances in biochemical parameters, gene expression, and histological picture of the liver and pancreas of diabetic rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 2829–2843. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.J.; Liao, S.R.; Huang, W.X.; Huang, C.; Liu, H.S.; Shen, W.Z. Intermittent fasting therapy promotes insulin sensitivity by inhibiting NLRP3 inflammasome in rat model. Ann. Palliat. Med. 2021, 10, 5299–5309. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, A.M.; Adedapo, A.D.A.; Badaki, V.B.; Oyagbemi, A.A.; Adedapo, A.A. Chrysophyllum albidum fruit ethanol extract ameliorates hyperglycaemia and elevated blood pressure in streptozotocin-induced diabetic rats through modulation of oxidative stress, NF-κB and PPAR-γ. Biomed. Pharmacother. 2021, 141, 111879. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Lo, Y.M.; Chang, W.C.; Lin, C.Y.; Chen, J.A.; Wu, J.S.; Huang, W.C.; Shen, S.C. Alleviative effect of Ruellia tuberosa L. on NAFLD and hepatic lipid accumulation via modulating hepatic de novo lipogenesis in high-fat diet plus streptozotocin-induced diabetic rats. Food Sci. Nutr. 2020, 8, 5710–5716. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.R.; Jothi, G.; Antony, P.J.; Balakrishna, K.; Paulraj, M.G.; Ignacimuthu, S.; Stalin, A.; Al-Dhabi, N.A. Gallic acid attenuates high-fat diet fed-streptozotocin-induced insulin resistance via partial agonism of PPARγ in experimental type 2 diabetic rats and enhances glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway. Eur. J. Pharmacol. 2014, 745, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tang, D.; Zhao, H.; Xin, X.; Aisa, H.A. Hypoglycemic effect of the polyphenols rich extract from Rose rugosa Thunb on high fat diet and STZ induced diabetic rats. J. Ethnopharmacol. 2017, 200, 174–181. [Google Scholar] [CrossRef]
- Antony, P.J.; Gandhi, G.R.; Stalin, A.; Balakrishna, K.; Toppo, E.; Sivasankaran, K.; Ignacimuthu, S.; Al-Dhabi, N.A. Myoinositol ameliorates high-fat diet and streptozotocin-induced diabetes in rats through promoting insulin receptor signaling. Biomed. Pharmacother. 2017, 88, 1098–1113. [Google Scholar] [CrossRef] [PubMed]
- Stalin, A.; Kandhasamy, S.; Kannan, B.S.; Verma, R.S.; Ignacimuthu, S.; Kim, Y.; Shao, Q.; Chen, Y.; Palani, P. Synthesis of a 1,2,3-bistriazole derivative of embelin and evaluation of its effect on high-fat diet fed-streptozotocin-induced type 2 diabetes in rats and molecular docking studies. Bioorg. Chem. 2020, 96, 103579. [Google Scholar] [CrossRef] [PubMed]
- Karganov, M.Y.; Alchinova, I.B.; Tinkov, A.A.; Medvedeva, Y.S.; Lebedeva, M.A.; Ajsuvakova, O.P.; Polyakova, M.V.; Skalnaya, M.G.; Burtseva, T.I.; Notova, S.V.; et al. Streptozotocin (STZ)-Induced Diabetes Affects Tissue Trace Element Content in Rats in a Dose-Dependent Manner. Biol. Trace Elem. Res. 2020, 198, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, R.; Liu, B.; Wang, W.; Yang, P.; Cao, Z.; Yang, X.; Du, W.; Yang, Q.; Liang, J.; et al. Antidiabetic Effect of Rehmanniae Radix Based on Regulation of TRPV1 and SCD1. Front. Pharmacol. 2022, 13, 875014. [Google Scholar] [CrossRef] [PubMed]
- Abdulmalek, S.; Eldala, A.; Awad, D.; Balbaa, M. Ameliorative effect of curcumin and zinc oxide nanoparticles on multiple mechanisms in obese rats with induced type 2 diabetes. Sci. Rep. 2021, 11, 20677. [Google Scholar] [CrossRef]
- Kolefer, K.; Miaffo, D.; Ponka, R. Evaluation of Antidiabetic Properties of the Leaves Extract of Ficus vallis-choudae Delile in a Model of Type 2 Diabetes Induced by High-Fat Diet and Streptozotocin. Sci. World J. 2021, 2021, 1502230. [Google Scholar] [CrossRef] [PubMed]
- Dubey, H.; Dubey, A.; Gulati, K.; Ray, A. Protective effects of L-arginine on cognitive deficits and biochemical parameters in an experimental model of type-2 diabetes mellitus induced Alzheimer’s disease in rats. J. Physiol. Pharmacol. 2022, 73, 3–17. [Google Scholar] [CrossRef]
- Sahin, K.; Onderci, M.; Tuzcu, M.; Ustundag, B.; Cikim, G.; Ozercan, I.H.; Sriramoju, V.; Juturu, V.; Komorowski, J.R. Effect of chromium on carbohydrate and lipid metabolism in a rat model of type 2 diabetes mellitus: The fat-fed, streptozotocin-treated rat. Metabolism 2007, 56, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Parveen, K.; Khan, M.R.; Mujeeb, M.; Siddiqui, W.A. Protective effects of Pycnogenol on hyperglycemia-induced oxidative damage in the liver of type 2 diabetic rats. Chem. Biol. Interact. 2010, 186, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.R.; Niture, N.T.; Ansari, A.A.; Shah, P.D. Anti-diabetic activity of embelin: Involvement of cellular inflammatory mediators, oxidative stress and other biomarkers. Phytomedicine 2013, 20, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Momtazi-Borojeni, A.A.; Jaafari, M.R.; Abdollahi, E.; Banach, M.; Sahebkar, A. Impact of PCSK9 Immunization on Glycemic Indices in Diabetic Rats. J. Diabetes Res. 2021, 2021, 4757170. [Google Scholar] [CrossRef] [PubMed]
- Karunanayake, E.H.; Hearse, D.J.; Mellows, G. Streptozotocin: Its excretion and metabolism in the rat. Diabetologia 1976, 12, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Shawky, L.M.; Morsi, A.A.; El Bana, E.; Hanafy, S.M. The Biological Impacts of Sitagliptin on the Pancreas of a Rat Model of Type 2 Diabetes Mellitus: Drug Interactions with Metformin. Biology 2019, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Tékus, V.; Horváth, Á.; Csekő, K.; Szabadfi, K.; Kovács-Valasek, A.; Dányádi, B.; Deres, L.; Halmosi, R.; Sághy, É.; Varga, Z.V.; et al. Protective effects of the novel amine-oxidase inhibitor multi-target drug SZV 1287 on streptozotocin-induced beta cell damage and diabetic complications in rats. Biomed. Pharmacother. 2021, 134, 111105. [Google Scholar] [CrossRef] [PubMed]
- Altındağ, F.; Rağbetli, M.; Özdek, U.; Koyun, N.; Ismael Alhalboosi, J.K.; Elasan, S. Combined treatment of sinapic acid and ellagic acid attenuates hyperglycemia in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2021, 156, 112443. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Deng, L.; Xiong, H.; Li, G.; Lin, J.; Liu, S.; Xie, J.; Liu, J.; Kong, F.; Tu, G.; et al. Expression profile of mitrogen-activated protein kinase (MAPK) signaling genes in the skeletal muscle & liver of rat with type 2 diabetes: Role in disease pathology. Indian J. Med. Res. 2014, 140, 744–755. [Google Scholar]
- Hayashi, Y.; Kajimoto, K.; Iida, S.; Sato, Y.; Mizufune, S.; Kaji, N.; Kamiya, H.; Baba, Y.; Harashima, H. DNA microarray analysis of whole blood cells and insulin-sensitive tissues reveals the usefulness of blood RNA profiling as a source of markers for predicting type 2 diabetes. Biol. Pharm. Bull. 2010, 33, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, Y.; Hu, J.; Liu, S.; Zhou, Z.; Xie, L. Lipid metabolism in type 1 diabetes mellitus: Pathogenetic and therapeutic implications. Front. Immunol. 2022, 13, 999108. [Google Scholar] [CrossRef] [PubMed]
- Niesen, M.; Bedi, M.; Lopez, D. Diabetes alters LDL receptor and PCSK9 expression in rat liver. Arch. Biochem. Biophys. 2008, 470, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Sparks, J.D.; Zolfaghari, R.; Sparks, C.E.; Smith, H.C.; Fisher, E.A. Impaired hepatic apolipoprotein B and E translation in streptozotocin diabetic rats. J. Clin. Investig. 1992, 89, 1418–1430. [Google Scholar] [CrossRef] [PubMed]
- Wanders, R.J. Metabolic functions of peroxisomes in health and disease. Biochimie 2014, 98, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro-Machado, E.; Gurgul-Convey, E.; Marzec, M.T. Immunometabolism in type 2 diabetes mellitus: Tissue-specific interactions. Arch. Med. Sci. 2023, 19, 895–911. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Uña, M.; López-Mancheño, Y.; Diéguez, C.; Fernández-Rojo, M.A.; Novelle, M.G. Unraveling the Role of Leptin in Liver Function and Its Relationship with Liver Diseases. Int. J. Mol. Sci. 2020, 21, 9368. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Wirtz, M.; Parker, N.; Hogan, M.; Strahler, J.; Michailidis, G.; Schmidt, S.; Vidal-Puig, A.; Diano, S.; Andrews, P.; et al. Leptin-mediated changes in hepatic mitochondrial metabolism, structure, and protein levels. Proc. Natl. Acad. Sci. USA 2009, 106, 13100–13105. [Google Scholar] [CrossRef] [PubMed]
- Paz-Filho, G.; Mastronardi, C.; Wong, M.L.; Licinio, J. Leptin therapy, insulin sensitivity, and glucose homeostasis. Indian J. Endocrinol. Metab. 2012, 16 (Suppl. S3), S549–S555. [Google Scholar] [CrossRef] [PubMed]
- Steiner, S.; Wahl, D.; Varela, M.C.; Aicher, L.; Prieto, P. Protein variability in male and female Wistar rat liver proteins. Electrophoresis 1995, 16, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Çelık, V.K.; Şahın, Z.D.; Sari, İ.; Bakir, S. Comparison of oxidant/antioxidant, detoxification systems in various tissue homogenates and mitochondria of rats with diabetes induced by streptozocin. Exp. Diabetes Res. 2012, 2012, 386831. [Google Scholar] [CrossRef] [PubMed]
- Arias-Loste, M.T.; Iruzubieta, P.; Puente, Á.; Ramos, D.; Santa Cruz, C.; Estébanez, Á.; Llerena, S.; Alonso-Martín, C.; San Segundo, D.; Álvarez, L.; et al. Increased Expression Profile and Functionality of TLR6 in Peripheral Blood Mononuclear Cells and Hepatocytes of Morbidly Obese Patients with Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2016, 17, 1878. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.O.; Correia, B.R.O.; Marinho, T.S.; Barbosa-da-Silva, S.; Mandarim-de-Lacerda, C.A.; Souza-Mello, V. Anti-steatotic linagliptin pleiotropic effects encompasses suppression of de novo lipogenesis and ER stress in high-fat-fed mice. Mol. Cell Endocrinol. 2020, 509, 110804. [Google Scholar] [CrossRef] [PubMed]
- Libby, A.E.; Bales, E.; Orlicky, D.J.; McManaman, J.L. Perilipin-2 Deletion Impairs Hepatic Lipid Accumulation by Interfering with Sterol Regulatory Element-binding Protein (SREBP) Activation and Altering the Hepatic Lipidome. J. Biol. Chem. 2016, 291, 24231–24246. [Google Scholar] [CrossRef] [PubMed]
- Engelking, L.J.; Kuriyama, H.; Hammer, R.E.; Horton, J.D.; Brown, M.S.; Goldstein, J.L.; Liang, G. Overexpression of Insig-1 in the livers of transgenic mice inhibits SREBP processing and reduces insulin-stimulated lipogenesis. J. Clin. Investig. 2004, 113, 1168–1175. [Google Scholar] [CrossRef]
- Kotani, K.; Peroni, O.D.; Minokoshi, Y.; Boss, O.; Kahn, B.B. GLUT4 glucose transporter deficiency increases hepatic lipid production and peripheral lipid utilization. J. Clin. Investig. 2004, 114, 1666–1675. [Google Scholar] [CrossRef]
- Gao, L.; Sun, N.; Xu, Q.; Jiang, Z.; Li, C. Comparative analysis of mRNA expression profiles in Type 1 and Type 2 diabetes mellitus. Epigenomics 2019, 11, 685–699. [Google Scholar] [CrossRef]
- Rönn, T.; Ofori, J.K.; Perfilyev, A.; Hamilton, A.; Pircs, K.; Eichelmann, F.; Garcia-Calzon, S.; Karagiannopoulos, A.; Stenlund, H.; Wendt, A.; et al. Genes with epigenetic alterations in human pancreatic islets impact mitochondrial function, insulin secretion, and type 2 diabetes. Nat. Commun. 2023, 14, 8040. [Google Scholar] [CrossRef]
- Hassan, M.A.; Elmageed, G.M.A.; El-Qazaz, I.G.; El-Sayed, D.S.; El-Samad, L.M.; Abdou, H.M. The Synergistic Influence of Polyflavonoids from Citrus aurantifolia on Diabetes Treatment and Their Modulation of the PI3K/AKT/FOXO1 Signaling Pathways: Molecular Docking Analyses and In Vivo Investigations. Pharmaceutics 2023, 15, 2306. [Google Scholar] [CrossRef]
- Sandoval-Muñíz, R.D.J.; Vargas-Guerrero, B.; Guzmán, T.J.; García-López, P.M.; Martínez-Ayala, A.L.; Domínguez-Rosales, J.A.; Gurrola-Díaz, C.M. Lupin gamma conglutin protein: Effect on Slc2a2, Gck and Pdx-1 gene expression and GLUT2 levels in diabetic rats. Rev. Bras. Farmacogn. 2018, 28, 716–723. [Google Scholar] [CrossRef]
- Magaña-Cerino, J.M.; Tiessen, A.; Soto-Luna, I.C.; Peniche-Pavía, H.A.; Vargas-Guerrero, B.; Domínguez-Rosales, J.A.; García-López, P.M.; Gurrola-Díaz, C.M. Consumption of nixtamal from a new variety of hybrid blue maize ameliorates liver oxidative stress and inflammation in a high-fat diet rat model. J. Funct. Foods 2020, 72, 104075. [Google Scholar] [CrossRef]
- Guerra-Ávila, P.L.; Guzmán, T.J.; Domínguez-Rosales, J.A.; García-López, P.M.; Cervantes-Garduño, A.B.; Wink, M.; Gurrola-Díaz, C.M. Combined Gamma Conglutin and Lupanine Treatment Exhibits In Vivo an Enhanced Antidiabetic Effect by Modulating the Liver Gene Expression Profile. Pharmaceuticals 2023, 16, 117. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef] [PubMed]







| Experimental Groups | ||||||
|---|---|---|---|---|---|---|
| Healthy Animals | HFD + STZ Model | STZ Model | ||||
| Initial Time | Final Time | Pre- Induction | Post- Induction | Pre- Induction | Post- Induction | |
| Glucose (mg/dL) | 125.0 ± 6.4 | 116.8 ± 8.8 | 169.6 ± 10.2 | 282.4 ± 46.6 * | 141.4 ± 5.2 | 215.6 ± 34.1 * |
| Triglycerides (mg/dL) | 53.0 ± 6.6 | 46.4 ± 4.7 | 77.6 ± 4.5 | 156.2 ± 33.9 | 67.6 ± 1.8 | 91.4 ± 6.5 * |
| Cholesterol (mg/dL) | 59.8 ± 10.5 | 57.8 ± 8.8 | 78.0 ± 3.4 | 171.3 ± 18.4 * | 50.0 ± 8.6 | 59.4 ± 10.5 |
| HDL-c (mg/dL) | 43.4 ± 10.4 | 42.3 ± 2.0 | 39.0 ± 2.8 | 41.8 ± 3.7 | 44.2 ± 1.6 | 49.5 ± 8.5 |
| LDL-c (mg/dL) | 5.8 ± 0.8 | 12.2 ± 7.8 | 18.9 ± 3.4 | 119.3 ± 16.9 | 15.4 ± 9.5 | 13.0 ± 7.3 |
| AST (U/L) | 58.7 ± 7.6 | 54.7 ± 2.7 | 63.0 ± 6.4 | 46.0 ± 8.9 | 58.7 ± 3.6 | 38.5 ± 4.7 |
| ALT (U/L) | 34.5 ± 3.2 | 29.6 ± 2.1 | 69.8 ± 17.1 | 75.3 ± 25.1 | 55.3 ± 15.5 | 29.0 ± 2.8 |
| Urea (mg/dL) | 46.6 ± 6.6 | 42.4 ± 2.0 | 55.6 ± 7.8 | 41.0 ± 5.2 | 58.0 ± 5.5 | 75.6 ± 4.8 * |
| Creatinine (mg/dL) | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.1 | 0.9 ± 0.1 * | 0.7 ± 0.1 | 0.6 ± 0.0 |
| Healthy vs. HFD + STZ | Healthy vs. STZ | HFD + STZ vs. STZ | |||
| Upregulated | |||||
| Gene | Fold Change | Gene | Fold Change | Gene | Fold Change |
| (log2) | (log2) | (log2) | |||
| Idi1 | 32.44 | Stac3 | 10.89 | Mmp12 | 36.32 |
| Msmo1 | 19.65 | Omd | 3.19 | Clec7a | 25.64 |
| Cyp51 | 17.87 | Car3 | 2.88 | Scd1 | 25 |
| Inmt | 17.07 | Omd | 2.87 | Lilrb4 | 23.21 |
| Sqle | 15.97 | Cdh17 | 2.87 | Gpnmb | 21.67 |
| Tm7sf2 | 14.35 | Tstd1 | 2.84 | Lpl | 21.35 |
| Stac3 | 12.03 | Scd1 | 2.67 | Spp1 | 16.45 |
| Hmgcs1 | 9.42 | Rps14 | 2.57 | Pla2g7 | 16.13 |
| Acss2 | 7.7 | Fabp7 | 2.38 | Scd2 | 10.84 |
| Hsd17b7 | 6.49 | Rps17l | 2.36 | Wfdc21 | 10.33 |
| Downregulated | |||||
| Gene | Fold Change | Gene | Fold Change | Gene | Fold Change |
| (log2) | (log2) | (log2) | |||
| Abcb1b | −33.97 | Abcc3 | −7.98 | Idi1 | −30.24 |
| Gpnmb | −32.99 | Cyp1a1 | −7.9 | Msmo1 | −29.6 |
| Mmp12 | −27.99 | Cyp17a1 | −6.72 | Sqle | −23.97 |
| Lpl | −23.83 | Acmsd | −6.71 | Cyp51 | −21.37 |
| Clec7a | −20.92 | Elovl6 | −5.68 | Elovl6 | −12.76 |
| Lilrb4 | −18.13 | Cyp2c12 | −5.3 | Hmgcs1 | −12.37 |
| Pla2g7 | −17.45 | Ppif | −4.87 | Tm7sf2 | −11.68 |
| Spp1 | −13.96 | Elovl6 | −4.47 | Hsd17b7 | −11.4 |
| Scd2 | −13.38 | Ppm1l | −4.39 | Prlr | −10.25 |
| Lcn2 | −11.99 | Prlr | −4.39 | Inmt | −9.49 |
| Gene Ontology (GO) Analysis | |||
| Term | Description | Strength (log 10) | False Discovery Rate (p Value) |
| Biological processes | |||
| GO:0010312 | Detoxification of zinc ion | 1.69 | 0.0452 |
| GO:0006695 | Cholesterol biosynthetic process | 1.15 | 6.31 × 10−7 |
| GO:0016126 | Sterol biosynthetic process | 1.12 | 2.26 × 10−7 |
| GO:0016125 | Sterol metabolic process | 0.96 | 8.27 × 10−12 |
| GO:0008203 | Cholesterol metabolic process | 0.95 | 1.12 × 10−10 |
| Molecular function | |||
| GO:0098809 | Nitrite reductase activity | 1.45 | 0.0454 |
| GO:0032934 | Sterol binding | 0.87 | 0.0099 |
| GO:0050660 | Flavin adenine dinucleotide binding | 0.71 | 0.0454 |
| GO:0016491 | Oxidoreductase activity | 0.43 | 3.16 × 10−5 |
| GO:0016740 | Transferase activity | 0.24 | 0.0013 |
| Cellular components | |||
| GO:0005778 | Peroxisomal membrane | 0.84 | 0.0050 |
| GO:0005777 | Peroxisome | 0.56 | 0.0287 |
| GO:0031968 | Organelle outer membrane | 0.48 | 0.0272 |
| GO:0031301 | Integral component of organelle membrane | 0.44 | 0.0048 |
| GO:0019866 | Organelle inner membrane | 0.38 | 0.0025 |
| Mammalian Phenotype Ontology (Monarch) | |||
| MP:0010161 | Decreased brain cholesterol level | 1.45 | 0.0167 |
| MP:0010026 | Decreased liver cholesterol level | 1.08 | 0.00085 |
| MP:0003983 | Decreased cholesterol level | 0.92 | 0.0020 |
| MP:0012776 | Abnormal liver cholesterol level | 0.88 | 0.0014 |
| MP:0003119 | Abnormal digestive system development | 0.74 | 0.0240 |
| KEGG pathways | |||
| mmu00100 | Steroid biosynthesis | 1.39 | 6.36 × 10−8 |
| mmu00920 | Sulfur metabolism | 1.25 | 0.0119 |
| mmu00900 | Terpenoid backbone biosynthesis | 1.03 | 0.0119 |
| mmu04950 | Maturity onset diabetes of the young | 0.96 | 0.0190 |
| mmu04979 | Cholesterol metabolism | 0.85 | 0.0119 |
| WikiPathways | |||
| WP103 | Cholesterol biosynthesis | 1.51 | 2.27 × 10−9 |
| WP4346 | Cholesterol metabolism with Bloch and Kandutsch−Russell pathways | 1.18 | 5.53 × 10−11 |
| WP1251 | Metapathway biotransformation | 0.66 | 0.00085 |
| WP447 | Adipogenesis genes | 0.57 | 0.0289 |
| Reactome Pathways | |||
| MMU-6807062 | Cholesterol biosynthesis via lathosterol | 1.57 | 0.0486 |
| MMU-6807047 | Cholesterol biosynthesis via desmosterol | 1.57 | 0.0486 |
| MMU-191273 | Cholesterol biosynthesis | 1.41 | 2.37 × 10−11 |
| MMU-9603798 | Class I peroxisomal membrane protein import | 1.09 | 0.0249 |
| MMU-8957322 | Metabolism of steroids | 0.9 | 6.59 × 10−9 |
| Gene Ontology (GO) Analysis | |||
| Term | Description | Strength (log 10) | False Discovery Rate (p Value) |
| Biological Processes | |||
| GO:0097527 | Necroptotic signaling pathway | 1.49 | 0.0258 |
| GO:0006002 | Fructose 6-phosphate metabolic process | 1.42 | 0.0057 |
| GO:0002467 | Germinal center formation | 1.4 | 0.0360 |
| GO:0002220 | Innate immune response activating cell surface receptor signaling pathway | 1.39 | 0.0014 |
| GO:0018158 | Protein oxidation | 1.36 | 0.0420 |
| Molecular Function | |||
| GO:0030246 | Carbohydrate binding | 0.75 | 3.93 × 10−5 |
| Cellular Components | |||
| GO:0009897 | External side of plasma membrane | 0.63 | 4.67 × 10−6 |
| GO:0098552 | Side of membrane | 0.53 | 3.65 × 10−5 |
| GO:0009986 | Cell surface | 0.51 | 1.40 × 10−6 |
| GO:0031984 | Organelle subcompartment | 0.35 | 0.0050 |
| GO:0005783 | Endoplasmic reticulum | 0.28 | 0.0115 |
| Mammalian Phenotype Ontology (Monarch) | |||
| MP:0011076 | Increased macrophage nitric oxide production | 1.79 | 0.0309 |
| MP:0008473 | Abnormal spleen follicular dendritic cell network | 1.74 | 0.0032 |
| MP:0020309 | Increased creatine kinase activity | 1.67 | 0.0425 |
| MP:0012448 | Abnormal primary motor cortex morphology | 1.67 | 0.0425 |
| MP:0008615 | Decreased circulating interleukin-17 level | 1.67 | 0.0425 |
| KEGG Pathways | |||
| mmu00524 | Neomycin, kanamycin and gentamicin biosynthesis | 1.74 | 0.0036 |
| mmu00052 | Galactose metabolism | 1.06 | 0.0127 |
| mmu00520 | Amino sugar and nucleotide sugar metabolism | 0.98 | 0.0089 |
| mmu05321 | Inflammatory bowel disease | 0.97 | 0.0036 |
| mmu04672 | Intestinal immune network for IgA production | 0.96 | 0.0248 |
| WikiPathways | |||
| WP4474 | Circulating monocytes and cardiac macrophages in diastolic dysfunction | 1.67 | 0.0472 |
| WP113 | TGF-beta signaling pathway | 1.17 | 3.65 × 10−5 |
| WP1253 | Type II interferon signaling (IFNG) | 1.08 | 0.0256 |
| WP3625 | Tyrobp causal network in microglia | 0.98 | 0.0042 |
| WP2432 | Spinal cord injury | 0.93 | 0.00023 |
| Reactome Pathways | |||
| MMU-5621480 | Dectin-2 family | 1.54 | 0.0448 |
| MMU-5668541 | TNFR2 non-canonical NF-kB pathway | 0.93 | 0.0011 |
| MMU-1280215 | Cytokine Signaling in Immune system | 0.6 | 0.0011 |
| MMU-168249 | Innate Immune System | 0.56 | 1.98 × 10−8 |
| MMU-6798695 | Neutrophil degranulation | 0.55 | 0.0011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra-Ávila, P.L.; Guzmán, T.J.; Vargas-Guerrero, B.; Domínguez-Rosales, J.A.; Cervantes-Garduño, A.B.; Salazar-Montes, A.M.; Sánchez-Orozco, L.V.; Gurrola-Díaz, C.M. Comparative Screening of the Liver Gene Expression Profiles from Type 1 and Type 2 Diabetes Rat Models. Int. J. Mol. Sci. 2024, 25, 4151. https://doi.org/10.3390/ijms25084151
Guerra-Ávila PL, Guzmán TJ, Vargas-Guerrero B, Domínguez-Rosales JA, Cervantes-Garduño AB, Salazar-Montes AM, Sánchez-Orozco LV, Gurrola-Díaz CM. Comparative Screening of the Liver Gene Expression Profiles from Type 1 and Type 2 Diabetes Rat Models. International Journal of Molecular Sciences. 2024; 25(8):4151. https://doi.org/10.3390/ijms25084151
Chicago/Turabian StyleGuerra-Ávila, Paloma Lucía, Tereso J. Guzmán, Belinda Vargas-Guerrero, José Alfredo Domínguez-Rosales, Alejandra Beatriz Cervantes-Garduño, Adriana María Salazar-Montes, Laura Verónica Sánchez-Orozco, and Carmen Magdalena Gurrola-Díaz. 2024. "Comparative Screening of the Liver Gene Expression Profiles from Type 1 and Type 2 Diabetes Rat Models" International Journal of Molecular Sciences 25, no. 8: 4151. https://doi.org/10.3390/ijms25084151
APA StyleGuerra-Ávila, P. L., Guzmán, T. J., Vargas-Guerrero, B., Domínguez-Rosales, J. A., Cervantes-Garduño, A. B., Salazar-Montes, A. M., Sánchez-Orozco, L. V., & Gurrola-Díaz, C. M. (2024). Comparative Screening of the Liver Gene Expression Profiles from Type 1 and Type 2 Diabetes Rat Models. International Journal of Molecular Sciences, 25(8), 4151. https://doi.org/10.3390/ijms25084151