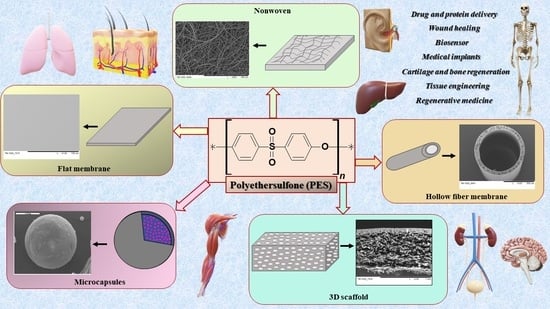
Polyethersulfone Polymer for Biomedical Applications and Biotechnology
Abstract
:1. Introduction
2. Characteristics of PES
3. The Application of PES in Biotechnology and Medicine
3.1. Scaffolds 3D
3.2. Hollow Fiber Membranes
3.3. Microcapsules
3.4. Flat Membranes
3.5. PES Nanofibers
4. Conclusions and Perspective
Funding
Conflicts of Interest
References
- Wypij, M.; Trzcińska-Wencel, J.; Golińska, P.; Avila-Quezada, G.D.; Ingle, A.P.; Rai, M. The strategic applications of natural polymer nanocomposites in food packaging and agriculture: Chances, challenges, and consumers’ perception. Front. Chem. 2023, 10, 1106230. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, M.M. Production of Polymer Hydrogel Composites and Their Applications. J. Polym. Environ. 2023, 31, 2855–2879. [Google Scholar] [CrossRef]
- Babu, P.J.; Tingirikari, J.M.R. A review on polymeric nanomaterials intervention in food industry. Polym. Bull. 2023, 80, 137–164. [Google Scholar] [CrossRef]
- Shahbaz, U.; Basharat, S.; Javed, U.; Bibi, A.; Yu, X. Bin Chitosan: A multipurpose polymer in food industry. Polym. Bull. 2023, 80, 3547–3569. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, J.; Chen, X.; Liu, W.; Chen, T. Cell Membrane Coating Technology: A Promising Strategy for Biomedical Applications; Springer: Singapore, 2019; Volume 11, ISBN 0123456789. [Google Scholar]
- Le, N.L.; Nunes, S.P. Materials and membrane technologies for water and energy sustainability. Sustain. Mater. Technol. 2016, 7, 1–28. [Google Scholar] [CrossRef]
- Xu, C.; Thiruvadi, V.S.; Whitmore, R.; Liu, H. Delivery systems for biomedical applications. In Biomaterials in Translational Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 93–116. [Google Scholar]
- Orive, G.; Emerich, D.; Khademhosseini, A.; Matsumoto, S.; Hernández, R.M.; Pedraz, J.L.; Desai, T.; Calafiore, R.; de Vos, P. Engineering a Clinically Translatable Bioartificial Pancreas to Treat Type I Diabetes. Trends Biotechnol. 2018, 36, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Gao, W.; Meng, F.; Liao, B.Q.; Leung, K.T.; Zhao, L.; Chen, J.; Hong, H. Membrane bioreactors for industrial wastewater treatment: A critical review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 677–740. [Google Scholar] [CrossRef]
- Farina, M.; Alexander, J.F.; Thekkedath, U.; Ferrari, M.; Grattoni, A. Cell encapsulation: Overcoming barriers in cell transplantation in diabetes and beyond. Adv. Drug Deliv. Rev. 2019, 139, 92–115. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue engineering approaches in the design of healthy and pathological in vitro tissue models. Front. Bioeng. Biotechnol. 2017, 5, 40. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in skin regeneration using tissue engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef]
- Mazza, G.; Al-Akkad, W.; Rombouts, K.; Pinzani, M. Liver Tissue Engineering: From Implantable Tissue to Whole organ engineering. Hepatol. Commun. 2018, 2, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Shin, Y.M.; Kim, K.; Shin, H. Tissue Engineering and Regenerative Medicine 2017: A Year in Review. Tissue Eng.—Part B Rev. 2018, 24, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.E.; Rodrigues, M.T.; Domingues, R.M.A.; Reis, R.L. Tissue engineering and regenerative medicine: New trends and directions—A year in review. Tissue Eng.—Part B Rev. 2017, 23, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—Different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef]
- Ahmadi, F.; Giti, R.; Mohammadi-Samani, S.; Mohammadi, F. Biodegradable Scaffolds for Cartilage Tissue Engineering. Galen Med. J. 2017, 6, 70–80. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and biocompatible polymers for tissue engineering application: A review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 185–192. [Google Scholar] [CrossRef]
- Zhao, T.; Wei, Z.; Zhu, W.; Weng, X. Recent Developments and Current Applications of Hydrogels in Osteoarthritis. Bioengineering 2022, 9, 132. [Google Scholar] [CrossRef]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: A review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed]
- Tajik, S.; Garcia, C.N.; Gillooley, S.; Tayebi, L. 3D Printing of Hybrid-Hydrogel Materials for Tissue Engineering: A Critical Review. Regen. Eng. Transl. Med. 2023, 9, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Wasyłeczko, M.; Sikorska, W.; Chwojnowski, A. Review of synthetic and hybrid scaffolds in cartilage tissue engineering. Membranes 2020, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef] [PubMed]
- Tsurumi, T.; Fuse, M. Enhancement of apatite precipitation on an alkaline hydrolyzed poly (lactic acid-ε-Caprolactone) film in simulated body fluid. J. Hard Tissue Biol. 2014, 23, 15–19. [Google Scholar] [CrossRef]
- Wasyłeczko, M.; Sikorska, W.; Przytulska, M.; Dulnik, J.; Chwojnowski, A. Polyester membranes as 3D scaffolds for cell culture. Desalination Water Treat. 2021, 214, 181–193. [Google Scholar] [CrossRef]
- Wasyłeczko, M.; Krysiak, Z.J.; Łukowska, E.; Gruba, M.; Sikorska, W.; Kruk, A.; Dulnik, J.; Czubak, J.; Chwojnowski, A. Three-dimensional scaffolds for bioengineering of cartilage tissue. Biocybern. Biomed. Eng. 2022, 42, 494–511. [Google Scholar] [CrossRef]
- Baranowski, M.; Wasyłeczko, M.; Kosowska, A.; Plichta, A.; Kowalczyk, S.; Chwojnowski, A.; Bielecki, W.; Czubak, J. Regeneration of Articular Cartilage Using Membranes of Polyester Scaffolds in a Rabbit Model. Pharmaceutics 2022, 14, 1016. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, M.S. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef]
- Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-roitman, J.; Schroeder, A. Mini Review Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Janmohammadi, M.; Nourbakhsh, M.S. Electrospun polycaprolactone scaffolds for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 527–539. [Google Scholar] [CrossRef]
- Dao, T.T.; Vu, N.B.; Pham, L.H.; Bui, T.; Le, P.T.; Van Pham, P. In Vitro Production of Cartilage Tissue from Rabbit Bone Marrow-Derived Mesenchymal Stem Cells and Polycaprolactone Scaffold. In Tissue Engineering and Regenerative Medicine; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Xu, Y.; Kim, C.; Saylor, D.M.; Koo, D. Review Article Polymer degradation and drug delivery in PLGA-based drug—Polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1692–1716. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Xie, Y.; Xiang, T.; Ma, L.; He, C.; Sun, S.D.; Zhao, C.S. Heparin-mimicking polyethersulfone membranes—Hemocompatibility, cytocompatibility, antifouling and antibacterial properties. J. Membr. Sci. 2016, 498, 135–146. [Google Scholar] [CrossRef]
- Mahboudi, H.; Soleimani, M.; Enderami, S.E.; Kehtari, M.; Hanaee-Ahvaz, H.; Ghanbarian, H.; Bandehpour, M.; Nojehdehi, S.; Mirzaei, S.; Kazemi, B. The effect of nanofibre-based polyethersulfone (PES) scaffold on the chondrogenesis of human induced pluripotent stem cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Dudziński, K.; Chwojnowski, A.; Gutowska, M.; Płończak, M.; Czubak, J.; Łukowska, E.; Wojciechowski, C. Three dimensional polyethersulphone scaffold for chondrocytes cultivation—The future supportive material for articular cartilage regeneration. Biocybern. Biomed. Eng. 2010, 30, 65–76. [Google Scholar]
- Plończak, M.; Czubak, J.; Hoser, G.; Chwojnowskl, A.; Kawiak, J.; Dudzińskp, K.; Czumińska, K. Repair of articular cartilage full thickness defects with cultured chondrocytes placed on polysulphonic membrane—Experimental studies in rabbits. Biocybern. Biomed. Eng. 2008, 28, 87–93. [Google Scholar]
- Irfan, M.; Idris, A. Overview of PES biocompatible/hemodialysis membranes: PES-blood interactions and modification techniques. Mater. Sci. Eng. C 2015, 56, 574–592. [Google Scholar] [CrossRef] [PubMed]
- Wasyłeczko, M.; Remiszewska, E.; Sikorska, W.; Dulnik, J.; Chwojnowskl, A. Scaffolds for Cartilage Tissue Engineering from a Blend of Polyethersulfone and Polyurethane Polymers. Molecules 2023, 28, 3195. [Google Scholar] [CrossRef]
- Płończak, M.; Wasyłeczko, M.; Jakutowicz, T.; Chwojnowski, A. Intraarticular Implantation of Autologous Chondrocytes Placed on Collagen or Polyethersulfone Scaffolds: An Experimental Study in Rabbits. Polymers 2023, 15, 2360. [Google Scholar] [CrossRef] [PubMed]
- Kaleekkal, N.J.; Thanigaivelan, A.; Tarun, M.; Mohan, D. A functional PES membrane for hemodialysis—Preparation, Characterization and Biocompatibility. Chin. J. Chem. Eng. 2015, 23, 1236–1244. [Google Scholar] [CrossRef]
- Athira, V.B.; Mohanty, S.; Nayak, S.K. Preparation and characterization of porous polyethersulfone (PES) membranes with improved biocompatibility by blending sulfonated polyethersulfone (SPES) and cellulose acetate (CA)—A comparative study. Mater. Today Commun. 2020, 25, 101544. [Google Scholar] [CrossRef]
- Hayama, M.; Yamamoto, K.I.; Kohori, F.; Sakai, K. How polysulfone dialysis membranes containing polyvinylpyrrolidone achieve excellent biocompatibility? J. Membr. Sci. 2004, 234, 41–49. [Google Scholar] [CrossRef]
- Ghaemi, N.; Daraei, P.; Akhlaghi, F.S. Polyethersulfone nanofiltration membrane embedded by chitosan nanoparticles: Fabrication, characterization and performance in nitrate removal from water. Carbohydr. Polym. 2018, 191, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Ravereau, J.; Fabre, A.; Brehant, A.; Bonnard, R.; Sollogoub, C.; Verdu, J. Ageing of polyvinylidene fluoride hollow fiber membranes in sodium hypochlorite solutions. J. Membr. Sci. 2016, 505, 174–184. [Google Scholar] [CrossRef]
- Yew, G.H.; Mohd Yusof, A.M.; Mohd Ishak, Z.A.; Ishiaku, U.S. Water absorption and enzymatic degradation of poly(lactic acid)/rice starch composites. Polym. Degrad. Stab. 2005, 90, 488–500. [Google Scholar] [CrossRef]
- Wu, C.S. Physical properties and biodegradability of maleated-polycaprolactone/starch composite. Polym. Degrad. Stab. 2003, 80, 127–134. [Google Scholar] [CrossRef]
- Zhao, C.; Xue, J.; Ran, F.; Sun, S. Modification of polyethersulfone membranes—A review of methods. Prog. Mater. Sci. 2013, 58, 76–150. [Google Scholar] [CrossRef]
- Zhao, C.; Tan, A.; Pastorin, G.; Ho, H.K. Nanomaterial scaffolds for stem cell proliferation and differentiation in tissue engineering. Biotechnol. Adv. 2013, 31, 654–668. [Google Scholar] [CrossRef]
- Ardeshirylajimi, A.; Dinarvand, P.; Seyedjafari, E.; Langroudi, L.; Jamshidi Adegani, F.; Soleimani, M. Enhanced reconstruction of rat calvarial defects achieved by plasma-treated electrospun scaffolds and induced pluripotent stem cells. Cell Tissue Res. 2013, 354, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Pournaqi, F.; Ghiaee, A.; Vakilian, S.; Ardeshirylajimi, A. Improved proliferation and osteogenic differentiation of mesenchymal stem cells on polyaniline composited by polyethersulfone nanofibers. Biologicals 2017, 45, 78–84. [Google Scholar] [CrossRef]
- Chu, Z.; Chen, K.; Xiao, C.; Ji, D.; Ling, H.; Li, M.; Liu, H. Improving pressure durability and fractionation property via reinforced PES loose nanofiltration hollow fiber membranes for textile wastewater treatment. J. Taiwan Inst. Chem. Eng. 2020, 108, 71–81. [Google Scholar] [CrossRef]
- Machodi, M.J.; Daramola, M.O. Synthesis and performance evaluation of PES/chitosan membranes coated with polyamide for acid mine drainage treatment. Sci. Rep. 2019, 9, 17657. [Google Scholar] [CrossRef] [PubMed]
- Simone, S.; Galiano, F.; Faccini, M.; Boerrigter, M.E.; Chaumette, C.; Drioli, E.; Figoli, A. Preparation and characterization of polymeric-hybrid PES/TiO2 hollow fiber membranes for potential applications in water treatment. Fibers 2017, 5, 14. [Google Scholar] [CrossRef]
- Thong, Z.; Gao, J.; Lim, J.X.Z.; Wang, K.Y.; Chung, T.S. Fabrication of loose outer-selective nanofiltration (NF) polyethersulfone (PES) hollow fibers via single-step spinning process for dye removal. Sep. Purif. Technol. 2018, 192, 483–490. [Google Scholar] [CrossRef]
- Bakeri, G.; Rezaei-DashtArzhandi, M.; Ismail, A.F.; Matsuura, T.; Abdullah, M.S.; Cheer, N.B. Porous polyethersulfone hollow fiber membrane in CO2 separation process via membrane contactor—The effect of nonsolvent additives. Korean J. Chem. Eng. 2017, 34, 160–169. [Google Scholar] [CrossRef]
- Shen, J.N.; Ruan, H.M.; Wu, L.G.; Gao, C.J. Preparation and characterization of PES-SiO2 organic-inorganic composite ultrafiltration membrane for raw water pretreatment. Chem. Eng. J. 2011, 168, 1272–1278. [Google Scholar] [CrossRef]
- Samtleben, W.; Dengler, C.; Reinhardt, B.; Nothdurft, A.; Lemke, H.D. Comparison of the new polyethersulfone high-flux membrane DIAPES® HF800 with conventional high-flux membranes during on-line haemodiafiltration. Nephrol. Dial. Transplant. 2003, 18, 2382–2386. [Google Scholar] [CrossRef]
- Guo, X.; Ma, Y.; Min, Y.; Sun, J.; Shi, X.; Gao, G.; Sun, L.; Wang, J. Progress and prospect of technical and regulatory challenges on tissue-engineered cartilage as therapeutic combination product. Bioact. Mater. 2023, 20, 501–518. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, X.; Wang, M.; Chen, J.; Zhang, A.; Gu, Z.; Zhao, C. BSA-modified polyethersulfone membrane: Preparation, characterization and biocompatibility. J. Biomater. Sci. Polym. Ed. 2009, 20, 377–397. [Google Scholar] [CrossRef] [PubMed]
- Gizer, G.; Önal, U.; Ram, M.; Sahiner, N. Biofouling and Mitigation Methods: A Review. Biointerface Res. Appl. Chem. 2023, 13, 1–25. [Google Scholar] [CrossRef]
- Alenazi, N.A.; Hussein, M.A.; Alamry, K.A.; Asiri, A.M. Modified polyether-sulfone membrane: A mini review. Des. Monomers Polym. 2017, 20, 532–546. [Google Scholar] [CrossRef]
- Ji, H.F.; Xiong, L.; Shi, Z.Q.; He, M.; Zhao, W.F.; Zhao, C.S. Engineering of hemocompatible and antifouling polyethersulfone membranes by blending with heparin-mimicking microgels. Biomater. Sci. 2017, 5, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Mollahosseini, A.; Abdelrasoul, A. Novel uremic metabolite-based zwitterionic polyethersulfone (PES) hemodialysis membrane for improved hemocompatibility: Towards the fourth generation of hemodialysis modifiers. Chem. Eng. Res. Des. 2023, 195, 466–479. [Google Scholar] [CrossRef]
- Abdelrasoul, A.; Shoker, A. Induced hemocompatibility of polyethersulfone (PES) hemodialysis membrane using polyvinylpyrrolidone: Investigation on human serum fibrinogen adsorption and inflammatory biomarkers released. Chem. Eng. Res. Des. 2022, 177, 615–624. [Google Scholar] [CrossRef]
- Li, F.; Meng, J.; Ye, J.; Yang, B.; Tian, Q.; Deng, C. Surface modification of PES ultrafiltration membrane by polydopamine coating and poly(ethylene glycol) grafting: Morphology, stability, and anti-fouling. Desalination 2014, 344, 422–430. [Google Scholar] [CrossRef]
- Liu, Y.; Koops, G.H.; Strathmann, H. Characterization of morphology controlled polyethersulfone hollow fiber membranes by the addition of polyethylene glycol to the dope and bore liquid solution. J. Membr. Sci. 2003, 223, 187–199. [Google Scholar] [CrossRef]
- Kalkan, R.; Nwekwo, C.W.; Adali, T. The Use of Scaffolds in Cartilage Regeneration. Eukaryot. Gene Expr. 2018, 28, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Marston, G.; Martin, A. Anatomy, Cartilage. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Walter, S.G.; Ossendorff, R.; Schildberg, F.A. Articular cartilage regeneration and tissue engineering models: A systematic review. Arch. Orthop. Trauma Surg. 2019, 139, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The role of tissue engineering in articular cartilage repair and regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, W.; Wasyłeczko, M.; Przytulska, M.; Wojciechowski, C.; Rokicki, G.; Chwojnowski, A. Chemical degradation of PSF-PUR blend hollow fiber membranes-assessment of changes in properties and morphology after hydrolysis. Membranes 2021, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, W.; Przytulska, M.; Wasyłeczko, M.; Wojciechowski, C.; Kulikowski, J.L.; Chwojnowski, A. Influence of hydrolysis, solvent and pvp addition on the porosity of psf/pur blend partly degradable hollow fiber membranes evaluated using the memoexplorer software. Desalination Water Treat. 2021, 214, 114–119. [Google Scholar] [CrossRef]
- Sikorska, W.; Milner-Krawczyk, M.; Wasyłeczko, M.; Wojciechowski, C.; Chwojnowski, A. Biodegradation process of PSF-PUR blend hollow fiber membranes using escherichia coli Bacteria—Evaluation of changes in properties and porosity. Polymers 2021, 13, 1311. [Google Scholar] [CrossRef]
- Sidikjanovna, T.D.; Qizi, H.G.N.; Ugli, N.M.G.; Ugli, F.S.F. Histological Structure of the Liver and Its Role in Complex Functions. Int. J. Eng. Inf. Syst. 2020, 4, 62–65. [Google Scholar]
- Ko, E.; Yoon, E.L.; Jun, D.W. Risk factors in nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S79–S85. [Google Scholar] [CrossRef] [PubMed]
- Berasain, C.; Arechederra, M.; Argemí, J.; Fernández-Barrena, M.G.; Avila, M.A. Loss of liver function in chronic liver disease: An identity crisis. J. Hepatol. 2023, 78, 401–414. [Google Scholar] [CrossRef]
- Pluta, K.D.; Ciezkowska, M.; Wisniewska, M.; Wencel, A.; Pijanowska, D.G. Cell-based clinical and experimental methods for assisting the function of impaired livers—Present and future of liver support systems. Biocybern. Biomed. Eng. 2021, 41, 1322–1346. [Google Scholar] [CrossRef]
- Kinasiewicz, A.; Dudzin, K.; Chwojnowski, A.; Weryn, A. Three-Dimensional Culture of Hepatocytes on Spongy Polyethersulfone Membrane Developed for Cell Transplantation. Transplant. Proc. 2007, 2916, 2914–2916. [Google Scholar] [CrossRef] [PubMed]
- Kinasiewicz, A.; Śmietanka, A.; Dudziński, K.; Chwojnowski, A.; Gajkowska, B.; Weryński, A. Spongy polyethersulfone membrane for hepatocyte cultivation: Studies on human hepatoma C3A cells. Artif. Organs 2008, 32, 747–752. [Google Scholar] [CrossRef]
- Jonkers, W.A.; Cornelissen, E.R.; de Vos, W.M. Hollow fiber nanofiltration: From lab-scale research to full-scale applications. J. Membr. Sci. 2023, 669, 121234. [Google Scholar] [CrossRef]
- Chwojnowski, A.; Wojciechowski, C.; Dudziński, K.; Łukowska, E. Polysulphone and polyethersulphone hollow fiber membranes with developed inner surface as material for bio-medical applications. Biocybern. Biomed. Eng. 2009, 29, 47–59. [Google Scholar]
- Unger, R.E.; Huang, Q.; Peters, K.; Protzer, D.; Paul, D.; Kirkpatrick, C.J. Growth of human cells on polyethersulfone (PES) hollow fiber membranes. Biomaterials 2005, 26, 1877–1884. [Google Scholar] [CrossRef]
- Modi, A.; Verma, S.K.; Bellare, J. Hydrophilic ZIF-8 decorated GO nanosheets improve biocompatibility and separation performance of polyethersulfone hollow fiber membranes: A potential membrane material for bioartificial liver application. Mater. Sci. Eng. C 2018, 91, 524–540. [Google Scholar] [CrossRef] [PubMed]
- Hakim, R.M. Hemodialysis. In National Kidney Foundation’s Primer on Kidney Diseases, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 508–519. [Google Scholar] [CrossRef]
- Ramada, D.L.; de Vries, J.; Vollenbroek, J.; Noor, N.; ter Beek, O.; Mihăilă, S.M.; Wieringa, F.; Masereeuw, R.; Gerritsen, K.; Stamatialis, D. Portable, wearable and implantable artificial kidney systems: Needs, opportunities and challenges. Nat. Rev. Nephrol. 2023, 19, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Na, D.E.C.; Hipertensiva, C. Hollow Fibers and Nanofibers in Membrane Science: Preparation, Characterization, and Applications; Alberto, F., Dorraji, M.S.S., Galiano, F., Eds.; Jenny Stanford: Dubai, United Arab Emirates, 2022; ISBN 9789814968034. [Google Scholar]
- Zheng, X.; Ni, C.; Xiao, W.; Yu, G.; Li, Y. In vitro hemocompatibility and hemodialysis performance of hydrophilic ionic liquid grafted polyethersulfone hollow fiber membranes. Sep. Purif. Technol. 2022, 298, 121464. [Google Scholar] [CrossRef]
- Fukuda, M.; Saomoto, H.; Shimizu, T.; Namekawa, K.; Sakai, K. Observation and proposed measurements of three-dimensional tortuous capillary pores with depth for hollow fiber hemoconcentrator membrane using dynamic force microscopy. Adv. Biomed. Eng. 2019, 8, 145–152. [Google Scholar] [CrossRef]
- PES Hollow Fiber Dialyzer (High-Flux Series). Available online: www.peony-medical.com (accessed on 8 April 2024).
- Said, N.; Lau, W.J.; Ho, Y.C.; Lim, S.K.; Zainol Abidin, M.N.; Ismail, A.F. A review of commercial developments and recent laboratory research of dialyzers and membranes for hemodialysis application. Membranes 2021, 11, 767. [Google Scholar] [CrossRef]
- Maduell, F.; Broseta, J.J.; Rodríguez-Espinosa, D.; Del Risco-Zevallos, J.; Gómez, M.; Rodas, L.M.; Arias-Guillén, M.; Vera, M.; Fontseré, N.; Salgado, M.D.C.; et al. Efficacy and Safety of the Medium Cut-Off Elisio HX Dialyzer. Blood Purif. 2023, 52, 68–74. [Google Scholar] [CrossRef] [PubMed]
- ter Beek, O.; Pavlenko, D.; Suck, M.; Helfrich, S.; Bolhuis-Versteeg, L.; Snisarenko, D.; Causserand, C.; Bacchin, P.; Aimar, P.; van Oerle, R.; et al. New Membranes Based on Polyethersulfone—SlipSkinTM Polymer Blends with Low Fouling and High Blood Compatibility; Elsevier B.V.: Amsterdam, The Netherlands, 2019; Volume 225, ISBN 3153489467. [Google Scholar]
- Verma, S.K.; Modi, A.; Bellare, J. Polyethersulfone-carbon nanotubes composite hollow fiber membranes with improved biocompatibility for bioartificial liver. Colloids Surf. B Biointerfaces 2019, 181, 890–895. [Google Scholar] [CrossRef]
- Ahmed, H.M.M.; Salerno, S.; Piscioneri, A.; Khakpour, S.; Giorno, L.; De Bartolo, L. Human liver microtissue spheroids in hollow fiber membrane bioreactor. Colloids Surf. B Biointerfaces 2017, 160, 272–280. [Google Scholar] [CrossRef]
- Unger, R.E.; Peters, K.; Huang, Q.; Funk, A.; Paul, D.; Kirkpatrick, C.J. Vascularization and gene regulation of human endothelial cells growing on porous polyethersulfone (PES) hollow fiber membranes. Biomaterials 2005, 26, 3461–3469. [Google Scholar] [CrossRef]
- Arsenijevic, T.; Perret, J.; Van Laethem, J.L.; Delporte, C. Aquaporins involvement in pancreas physiology and in pancreatic diseases. Int. J. Mol. Sci. 2019, 20, 5052. [Google Scholar] [CrossRef]
- Röder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef]
- Elsayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef]
- Figliuzzi, M.; Cornolti, R.; Plati, T.; Rajan, N.; Adobati, F.; Remuzzi, G.; Remuzzi, A. Subcutaneous xenotransplantation of bovine pancreatic islets. Biomaterials 2005, 26, 5640–5647. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Primers 2022, 2, 94. [Google Scholar] [CrossRef]
- de Jongh, D.; Massey, E.K.; Berishvili, E.; Fonseca, L.M.; Lebreton, F.; Bellofatto, K.; Bignard, J.; Seissler, J.; van Buerck, L.W.; Honarpisheh, M.; et al. Organoids: A systematic review of ethical issues. Stem Cell Res. Ther. 2022, 13, 337. [Google Scholar] [CrossRef]
- Houtekamer, R.M.; van der Net, M.C.; Maurice, M.M.; Gloerich, M. Mechanical forces directing intestinal form and function. Curr. Biol. 2022, 32, R791–R805. [Google Scholar] [CrossRef]
- Taelman, J.; Diaz, M.; Guiu, J. Human Intestinal Organoids: Promise and Challenge. Front. Cell Dev. Biol. 2022, 10, 854740. [Google Scholar] [CrossRef]
- Jochems, P.G.M.; van Bergenhenegouwen, J.; van Genderen, A.M.; Eis, S.T.; Wilod Versprille, L.J.F.; Wichers, H.J.; Jeurink, P.V.; Garssen, J.; Masereeuw, R. Development and validation of bioengineered intestinal tubules for translational research aimed at safety and efficacy testing of drugs and nutrients. Toxicol. Vitr. 2019, 60, 1–11. [Google Scholar] [CrossRef]
- Li, Q.; Shao, X.; Dai, X.; Guo, Q.; Yuan, B.; Liu, Y.; Jiang, W. Recent trends in the development of hydrogel therapeutics for the treatment of central nervous system disorders. NPG Asia Mater. 2022, 14, 14. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood–brain barrier breakdown in Alzheimer’s disease and other.pdf. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Doblado, L.R.; Martínez-Ramos, C.; Pradas, M.M. Biomaterials for Neural Tissue Engineering. Front. Nanotechnol. 2021, 3, 643507. [Google Scholar] [CrossRef]
- Wahlberg, L.U.; Lind, G.; Almqvist, P.M.; Kusk, P.; Tornøe, J.; Juliusson, B.; Söderman, M.; Selldén, E.; Seiger, Å.; Eriksdotter-Jönhagen, M.; et al. Targeted delivery of nerve growth factor via encapsulated cell biodelivery in Alzheimer disease: A technology platform for restorative neurosurgery—Clinical article. J. Neurosurg. 2012, 117, 340–347. [Google Scholar] [CrossRef]
- Eyjolfsdottir, H.; Eriksdotter, M.; Linderoth, B.; Lind, G.; Juliusson, B.; Kusk, P.; Almkvist, O.; Andreasen, N.; Blennow, K.; Ferreira, D.; et al. Targeted delivery of nerve growth factor to the cholinergic basal forebrain of Alzheimer’s disease patients: Application of a second-generation encapsulated cell biodelivery device. Alzheimer’s Res. Ther. 2016, 8, 30. [Google Scholar] [CrossRef]
- Pandey, S.; Biswas, G. An Overview on Microspheres and Microcapsules as Promising Drug Carriers. PriMera Sci. Surg. Res. Pract. 2023, 2, 3–15. [Google Scholar] [CrossRef]
- Ladeira, B.M.; Custódio, C.A.; Mano, J.F. Core-shell microcapsules: Biofabrication and potential applications in tissue engineering and regenerative medicine. Biomater. Sci. 2022, 10, 2122–2153. [Google Scholar] [CrossRef]
- Kupikowska-Stobba, B.; Lewińska, D. Polymer microcapsules and microbeads as cell carriers for: In vivo biomedical applications. Biomater. Sci. 2020, 8, 1536–1574. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Zhao, D.; Liu, M.; Hong, Z.; Liu, J.; Dai, K.; Xiao, X. Docking Design of the Different Microcapsules in Aqueous Solution and Its Quantitative On-Off Study. Polymers 2023, 15, 1131. [Google Scholar] [CrossRef]
- Kupikowska-Stobba, B.; Grzeczkowicz, M.; Lewińska, D. A one-step in vitro continuous flow assessment of protein release from core-shell polymer microcapsules designed for therapeutic protein delivery. Biocybern. Biomed. Eng. 2021, 41, 1347–1364. [Google Scholar] [CrossRef]
- Djekić, L.; Ćirić, A. Modeling of in vitro drug release from polymeric microparticle carriers. Arh. Farm. 2022, 72, 591–620. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019, 28, 801–812. [Google Scholar] [CrossRef]
- Mori, T.; Igarashi, M.; Onodera, Y.; Takehara, T.; Itokazu, M.; Teramura, T. Fibrinogen supports self-renewal of mesenchymal stem cells under serum-reduced condition through autophagy activation. Biochem. Biophys. Res. Commun. 2023, 651, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, K.; Nibbelink, M.G.; Karbaat, L.P.; Karperien, M.; van Apeldoorn, A.; Stamatialis, D. An important step towards a prevascularized islet macroencapsulation device—Effect of micropatterned membranes on development of endothelial cell network. J. Mater. Sci. Mater. Med. 2018, 29, 91. [Google Scholar] [CrossRef]
- Schophuizen, C.M.S.; De Napoli, I.E.; Jansen, J.; Teixeira, S.; Wilmer, M.J.; Hoenderop, J.G.J.; Van Den Heuvel, L.P.W.; Masereeuw, R.; Stamatialis, D. Development of a living membrane comprising a functional human renal proximal tubule cell monolayer on polyethersulfone polymeric membrane. Acta Biomater. 2015, 14, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Yeo, G.C.; Kondyurin, A.; Kosobrodova, E.; Weiss, A.S.; Bilek, M.M.M. A sterilizable, biocompatible, tropoelastin surface coating immobilized by energetic ion activation. J. R. Soc. Interface 2017, 14, 20160837. [Google Scholar] [CrossRef]
- Zailani, M.Z.; Ismail, A.F.; Sheikh Abdul Kadir, S.H.; Othman, M.H.D.; Goh, P.S.; Hasbullah, H.; Abdullah, M.S.; Ng, B.C.; Kamal, F. Hemocompatibility evaluation of poly(1,8-octanediol citrate) blend polyethersulfone membranes. J. Biomed. Mater. Res.—Part A 2017, 105, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, P.; Lei, B. Engineering multifunctional bioactive citrate-based biomaterials for tissue engineering. Bioact. Mater. 2023, 19, 511–537. [Google Scholar] [CrossRef] [PubMed]
- Intrapur Neonet. Available online: https://www.bbraun.pl/pl/products/b/intrapur-neonat.html (accessed on 8 April 2024).
- Intrapur Lipid. Available online: https://www.bbraun.pl/pl/products/b/intrapur-lipid.html (accessed on 8 April 2024).
- PureFlo ® Z Series PES. Available online: https://www.biopharm.saint-gobain.com/components/filtration (accessed on 8 April 2024).
- PES Membranes for Filtration Applications. Available online: https://www.porex.com/porous-polymers-technologies/porous-membranes/pes-membranes/ (accessed on 8 April 2024).
- Wang, C.; Wang, J.; Zeng, L.; Qiao, Z.; Liu, X.; Liu, H.; Zhang, J.; Ding, J. Fabrication of electrospun polymer nanofibers with diverse morphologies. Molecules 2019, 24, 834. [Google Scholar] [CrossRef]
- Ghajarieh, A.; Habibi, S.; Talebian, A. Biomedical Applications of Nanofibers. Russ. J. Appl. Chem. 2021, 94, 847–872. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.; Li, Y.; Tan, Y.; Liu, Y.; Pan, W.; Tan, G. Stimuli-responsive electrospun nanofibers for drug delivery, cancer therapy, wound dressing, and tissue engineering. J. Nanobiotechnology 2023, 21, 237. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; He, J.H. Bipolymer nanofibers: Engineering nanoscale interface via bubble electrospinning. J. Appl. Polym. Sci. 2024, 141, e54878. [Google Scholar] [CrossRef]
- Zheng, Q.; Xi, Y.; Weng, Y. Functional electrospun nanofibers: Fabrication, properties, and applications in wound-healing process. RSC Adv. 2024, 14, 3359–3378. [Google Scholar] [CrossRef] [PubMed]
- Venmathi Maran, B.A.; Jeyachandran, S.; Kimura, M. A Review on the Electrospinning of Polymer Nanofibers and Its Biomedical Applications. J. Compos. Sci. 2024, 8, 32. [Google Scholar] [CrossRef]
- Shabani, I.; Haddadi-Asl, V.; Soleimani, M.; Seyedjafari, E.; Babaeijandaghi, F.; Ahmadbeigi, N. Enhanced infiltration and biomineralization of stem cells on collagen-grafted three-dimensional nanofibers. Tissue Eng.—Part A 2011, 17, 1209–1218. [Google Scholar] [CrossRef]
- Moradi, S.L.; Golchin, A.; Hajishafieeha, Z.; Khani, M.M.; Ardeshirylajimi, A. Bone tissue engineering: Adult stem cells in combination with electrospun nanofibrous scaffolds. J. Cell. Physiol. 2018, 233, 6509–6522. [Google Scholar] [CrossRef]
- Ardeshirylajimi, A.; Farhadian, S.; Adegani, F.J.; Mirzaei, S.; Zomorrod, M.S.; Langroudi, L.; Doostmohammadi, A.; Seyedjafari, E.; Soleimani, M. Enhanced osteoconductivity of polyethersulphone nanofibres loaded with bioactive glass nanoparticles in in vitro and in vivo models. Cell Prolif. 2015, 48, 455–464. [Google Scholar] [CrossRef]
- Mahboudi, H.; Kazemi, B.; Soleimani, M.; Hanaee-Ahvaz, H.; Ghanbarian, H.; Bandehpour, M.; Enderami, S.E.; Kehtari, M.; Barati, G. Enhanced chondrogenesis of human bone marrow mesenchymal Stem Cell (BMSC) on nanofiber-based polyethersulfone (PES) scaffold. Gene 2018, 643, 98–106. [Google Scholar] [CrossRef]
- Mansour, R.N.; Barati, G.; Soleimani, M.; Ghoraeian, P.; Nouri Aleagha, M.; Kehtari, M.; Mahboudi, H.; Hosseini, F.; Hassannia, H.; Abazari, M.F.; et al. Generation of high-yield insulin producing cells from human-induced pluripotent stem cells on polyethersulfone nanofibrous scaffold. Artif. Cells Nanomed. Biotechnol. 2018, 46, 733–739. [Google Scholar] [CrossRef]
- Maymand, M.M.; Soleimanpour-lichaei, H.R.; Ardeshirylajimi, A.; Soleimani, M.; Enderami, S.E.; Nojehdehi, S.; Behjati, F.; Salmani, M.K. Improvement of hepatogenic differentiation of iPS cells on an aligned polyethersulfone compared to random nanofibers. Artif. Cells Nanomed. Biotechnol. 2018, 46, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Ghollasi, M. Enhanced neural differentiation of human-induced pluripotent stem cells on aligned laminin-functionalized polyethersulfone nanofibers; a comparison between aligned and random fibers on neurogenesis. J. Biomed. Mater. Res. Part A 2022, 110, 672–683. [Google Scholar] [CrossRef]
- Meng, M.V. Reported failures of the polymer self-locking (Hem-o-lok) clip: Review of data from the Food and Drug Administration. J. Endourol. 2006, 20, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Grigore, N.; Pirvut, V.; Mihai, I.; Mitariu, S.I.C.; Sava, M.; Hasegan, A. Polymer ligating clips in urologic laparoscopic surgery. Mater. Plast. 2017, 54, 295–297. [Google Scholar] [CrossRef]
- Boicean, A.; Birlutiu, V.; Ichim, C.; Todor, S.B.; Hasegan, A.; Bacila, C.; Solomon, A.; Cristian, A.; Dura, H. Predictors of Post-ERCP Pancreatitis (P.E.P.) in Choledochal Lithiasis Extraction. J. Pers. Med. 2023, 13, 1356. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.J.; Bomman, S.; Krishnamoorthi, R.; Kozarek, R.A. Endoscopic retrograde cholangiopancreatography: Current practice and future research. World J. Gastrointest. Endosc. 2021, 13, 260–274. [Google Scholar] [CrossRef] [PubMed]













| PES Product | Application | Information |
|---|---|---|
| 3D scaffolds | Cartilage regeneration—cell culture and laboratory study | In vivo study [43] |
| In vitro study [30] | ||
| Laboratory study [42] | ||
| Liver tissue—cell culture | In vitro study [84] | |
| In vivo study [83] | ||
| HFMs | Kidneys—hemodialysis, blood purification application | In vitro study [92] |
| Commercial membrane Purema [93,94] | ||
| Commercial membrane ELISIO [95,96] | ||
| PES-SS2 membrane—in vitro study [97] | ||
| Liver tissue—cell culture | PES-carbon nanotubes composite HFMs—In vitro study with HepG2 [98] | |
| A three-dimensional liver tissue model—In vitro study with cryopreserved primary human hepatocytes [99] | ||
| Endothelial cell culture | In vitro study with human endothelial cell growth [100] | |
| Pancreas—pancreatic islets in diabetic | In vitro and in vivo study—subcutaneous xenotransplantation of pancreatic islets in diabetic rats [104] | |
| Intestinal organoids—promise for the development of new drugs, nutraceuticals, food ingredients, and toxins | In vitro study with Coco-2 cells—model of PES HFMs and ECM components [109] | |
| Natural tissue—promise implant in neurodegenerative disease | A clinical study with Implant NsGene A/S [113] and the second-generation implant, NsG0202.1 [114] | |
| Microcapsules | Drug delivery system—system for improving the feasibility of multi-drug delivery systems. | The system with two different docking units of microcapsules PES-g-PAAC and PES-g-PAAM [118] |
| Drug release from aqueous core microcapsules | Alg/PES microcapsules—the process of protein diffusion from the alginate hydrogel core, through the polymeric wall [119,120] | |
| Flat membrane | Stem cell culture | Fg-PES flat membrane as a versatile flat membrane for regenerative medicine [123] |
| Device for encapsulation islet | Thin semipermeable flat membranes PES/PVP with a micropattern [124] | |
| Bioartificial kidney—“living membrane” | In vitro study with flat PES membrane coated by a double layer of L-DOPA and Coll IV [125] | |
| Tissue engineering and regenerative medicine | Biomimetic platform—modified PES membrane for the irreversible immobilization of bioactive proteins designed for cell culture [126] | |
| Blood purification, hemodialysis application | M3 flat membrane of PES and POC blending—in vitro study [127,128] | |
| Nanofibers | Bone tissue engineering | The use of COL-PES nanofibers to heal and regenerate bone—in vitro study [139] |
| PES nanofibers coated with bioactive glass—in vitro and in vivo study [140,141] | ||
| Modified composite of PES/PANi—in vitro study [54,140] | ||
| Cartilage tissue engineering | PES nanofibers for chondrogenic differentiation of bone-marrow-derived MSCs—in vitro study [142] | |
| iPSCs cultured on a PES nanofibers—in vitro study [38,62] | ||
| Pancreatic islet—treatment of patients with type 1 diabetes mellitus | Differentiation of iPSCs towards pancreatic cells on modified PES nanofibers—in vitro study [143] | |
| Liver tissue engineering | The hepatogenic differentiation of iPSCs on modified PES/COL nanofibers—in vitro study [144] | |
| Natural tissue engineering | The modified PES–plasma–laminin nanofiber scaffold as a versatile biomaterial platform for inducing neuronal differentiation of iPSCs—in vitro study [145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasyłeczko, M.; Wojciechowski, C.; Chwojnowski, A. Polyethersulfone Polymer for Biomedical Applications and Biotechnology. Int. J. Mol. Sci. 2024, 25, 4233. https://doi.org/10.3390/ijms25084233
Wasyłeczko M, Wojciechowski C, Chwojnowski A. Polyethersulfone Polymer for Biomedical Applications and Biotechnology. International Journal of Molecular Sciences. 2024; 25(8):4233. https://doi.org/10.3390/ijms25084233
Chicago/Turabian StyleWasyłeczko, Monika, Cezary Wojciechowski, and Andrzej Chwojnowski. 2024. "Polyethersulfone Polymer for Biomedical Applications and Biotechnology" International Journal of Molecular Sciences 25, no. 8: 4233. https://doi.org/10.3390/ijms25084233
APA StyleWasyłeczko, M., Wojciechowski, C., & Chwojnowski, A. (2024). Polyethersulfone Polymer for Biomedical Applications and Biotechnology. International Journal of Molecular Sciences, 25(8), 4233. https://doi.org/10.3390/ijms25084233