Performance of a Yeast-mediated Biological Fuel Cell
Abstract
:1. Introduction
2. Materials and Method
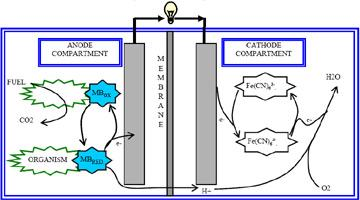
2.1. Construction of the Fuel Cell
2.2. Operation of the Fuel Cell
3. Results and Discussion
3.1. Variation of Open Circuit Voltage
3.2. Fuel Cell Behavior under Load
3.3. Fuel cell efficiency
4. Concluding Remarks
Acknowledgments
References
- Allen, MJ; Norris, JR; Ribbons, DW. Methods in Microbiology; Academic Press; New York, 1972; pp. 247–283. [Google Scholar]
- Allen, RM; Bennetto, HP. Microbial fuel-cells: Electricity production from carbohydrates. Appl. Biochem. Biotech. 1993, 39–40, 27–40. [Google Scholar]
- St-Pierre, J; Wilkinson, DP. Fuel cells: A new, efficient and cleaner power source. AIChE J 2001, 47, 1482–1486. [Google Scholar]
- Rabaey, K; Rodríguez, J; Blackall, LL; Keller, J; Gross, P; Batstone, D; Verstraete, W; Nealson, KH. Microbial ecology meets electrochemistry: Electricity-driven and driving communities. ISME J. 2007, 1, 9–18. [Google Scholar]
- Aiyuka, S; Forreza, I; Lievena, DK; van Haandelb, A; Verstraete, W. Anaerobic and complementary treatment of domestic sewage in regions with hot climates. Biores. Technol 2006, 97, 2225–2241. [Google Scholar]
- Rozendal, RA; Hamelers, HVM; Rabaey, K; Keller, J; Buisman, CJN. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 2008, 26, 450–459. [Google Scholar]
- Shukla, AK; Suresh, P; Berchmans, S; Rajendran, A. Biological fuel cells and their applications. Curr. Sci. India 2004, 87, 455–468. [Google Scholar]
- Stams, AJM; de Bok, FAM; Pligge, CM; van Eekert, MHA; Dolfing, J; Schraa, G. Exocellular electron tranasfer in anaerobic microbial communities. Environ. Microbio 2006, 8, 371–382. [Google Scholar]
- Gorby, YA; Yanina, S; McLean, JS; Rosso, KM; Moyles, D; Dohnalkova, A; Beveridge, TJ; Chang, IS; Kim, BH; Kim, KS; et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Nat. Acad. Sci. USA 2006, 103, 11358–11363. [Google Scholar]
- Reguera, G; McCarthy, KD; Mehta, T; Nicoll, JS; Tuominen, MT; Lovley, DR. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar]
- Newman, DK; Kolter, R. A role for excreted quinones in extracellular electron transfer. Nature 2000, 405, 94–97. [Google Scholar]
- Bond, DR; Loveley, DR. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microb 2003, 69, 1548–1555. [Google Scholar]
- Kim, BH; Park, HS; Kim, HJ; Kim, GT; Chang, IS; Lee, J; Phung, NT. Enrichment of microbial community generating electricity using a fuel-cell-type electrochemical cell. Appl. Microbiol. Biot. 2004, 63, 672–681. [Google Scholar]
- Kim, BH; Kim, HJ; Hyun, MS; Park, DH. Direct electrode reaction of Fe(III) -reducing bacterium, Shewanella putrefaciens. J. Microbiol. Biotechn 1999, 19, 127–131. [Google Scholar]
- Nelson, DL; Cox, MM. Principles of Biochemistry, 4th ed; W.H. Freeman: New York, 2005. [Google Scholar]
- Feldmann, H. Yeast Molecular Biology - A Short Compendium on Basic Features and Novel Aspects; Adolf-Butenandt-Institute, University of Munich: Munich, 2005. [Google Scholar]
- Barton, SC; Gallaway, J; Atanassov, P. Enzyatic Biofuel Cells for Implantable and Microscale Devices. Chem. Rev 2004, 104, 4867–4886. [Google Scholar]
- Lee, S-K; Mills, A. Novel photochemistry of leuco-Methylene Blue. Chem. Comm 2003, 2366–2367. [Google Scholar]
- Bard, AJ; Faulkner, LR. Electrochemical Methods: Fundamentals and Applications, 2nd Ed ed; John Wiley & Sons: New York, 2001. [Google Scholar]
- Barbir, F. PEM Fuel Cells: Theory and Practice; Elsevier Academic Press: San Diego, USA, 2005; pp. 33–63. [Google Scholar]
- Park, DH; Zeikus, G. Electricity generation in microbial fuel cells using neutral red as an electronophore. Appl. Environ. Microb 2000, 6, 1292–1297. [Google Scholar]
- Rabaey, K; Boon, N; Siciliano, SD; Verhaege, M; Verstraete, W. Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl. Environ. Microb. 2004, 70, 5373–5382. [Google Scholar]
- Park, DH; Kim, SK; Shin, IH; Jeong, YJ. Electricity production in biofuel cell using modified graphite electrode with Neutral Red. Biotechnol. Lett. 2000, 22, 1301–1304. [Google Scholar]
- Xiao, M; Wang, L; Information, C; Wu, Y; Huang, X; Dang, Z. Electrochemical study of methylene blue/titanate nanotubes nanocomposite and its layer-by-layer assembly multilayer films. J. Solid State Electr 2008, 12, 1159–1166. [Google Scholar]
- Dias, SLP; Fujiwara, ST; Gushikem, Y; Bruns, RE. Methylene blue immobilized on cellulose surfaces modified with titanium dioxide and titanium phosphate: factorial design optimization of redox properties. J. Electroanal. Chem. 2002, 531, 141–146. [Google Scholar]









| Exp. Number | Organism | Substrate | Mediator- (anode) | Catholyte |
|---|---|---|---|---|
| EX1 | Yeast | Glucose | Methylene blue | Methylene blue |
| EX2 | Yeast | Glucose | Methylene blue | K3Fe(CN)6 |
| EX3 | Yeast | Glucose | Methylene blue | Water |
| EX4 | Yeast | Glucose | Water | Water |
| EX5 | Yeast | Glucose | Water | Methylene blue |
| EX6 | Yeast | Glucose | Water | K3Fe(CN)6 |
| (a) | |
|---|---|
| Experiment | Reactions in the anode compartment |
| EX1 | MB(oxidized)+NADH(yeast) → NAD+(yeast) + MB(reduced) MB(reduced) → MB(oxidized) + 2e– + H+ |
| EX2 | MB(oxidized)+NADH(yeast) → NAD+(yeast) + MB(reduced) MB(reduced) → MB(oxidized) + 2e– + H+ |
| EX3 | MB(oxidized)+NADH(yeast) → NAD+(yeast) + MB(reduced) MB(reduced) → MB(oxidized) + 2e–+ H+ |
| EX4 | NADH(yeast) → NAD+(yeast) + H+ + e– |
| EX5 | NADH(yeast) → NAD+(yeast) + H+ + e– |
| EX6 | NADH(yeast) → NAD+(yeast) + H+ + e– |
| (b) | |
|---|---|
| Experiment | Reactions in the cathode compartment |
| EX1 | 4MB(oxidized) + 4H+ + 8e– → 4MB(reduced) 4MB(reduced) + O2 → 2H2O + 4MB(oxidized) |
| EX2 | 4PF(oxidized) + 4e– → 4PF(reduced) 4PF(reduced) + O2 + 4H+ → 2H2O + 4PF(oxidized) |
| EX3 | 4H+ + O2 + 4e– → 2H2O |
| EX4 | 4H+ + O2 + 4e– → 2H2O |
| EX5 | 4MB(oxidized)+4H+ + 8e– → 4MB(reduced) 4MB(reduced) + O2 → 2H2O + 4MB(oxidized) |
| EX6 | 4PF(oxidized) + 4e– → 4PF(reduced) 4PF(reduced) + O2 + 4H+ →2H2O + 4PF(oxidized) |
| Experiment No. | Open circuit Voltage-V (Theoretical) | Open circuit Voltage-V (Practical) |
|---|---|---|
| EX1 | 1.198 | 0.386 |
| EX2 | 0.858 | 0.332 |
| EX3 | 1.219 | 0.327 |
| EX4 | 1.209 | 0.234 |
| EX5 | 1.188 | 0.305 |
| EX6 | 0.848 | 0.209 |
| Loading pattern | Range | Increment |
|---|---|---|
| A | 400Ω–1000Ω | 100Ω |
| B | 1kΩ–10kΩ | 1kΩ |
| C | 10kΩ–100kΩ | 10kΩ |
© 2008 by MDPI This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gunawardena, A.; Fernando, S.; To, F. Performance of a Yeast-mediated Biological Fuel Cell. Int. J. Mol. Sci. 2008, 9, 1893-1907. https://doi.org/10.3390/ijms9101893
Gunawardena A, Fernando S, To F. Performance of a Yeast-mediated Biological Fuel Cell. International Journal of Molecular Sciences. 2008; 9(10):1893-1907. https://doi.org/10.3390/ijms9101893
Chicago/Turabian StyleGunawardena, Anuradh, Sandun Fernando, and Filip To. 2008. "Performance of a Yeast-mediated Biological Fuel Cell" International Journal of Molecular Sciences 9, no. 10: 1893-1907. https://doi.org/10.3390/ijms9101893
APA StyleGunawardena, A., Fernando, S., & To, F. (2008). Performance of a Yeast-mediated Biological Fuel Cell. International Journal of Molecular Sciences, 9(10), 1893-1907. https://doi.org/10.3390/ijms9101893