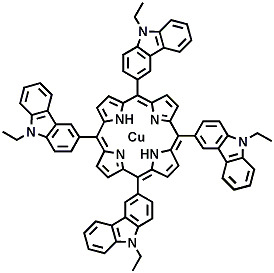
Copper 5,10,15,20-Tetra(N-ethyl-3-carbazolyl) Porphyrin
Abstract
:Introduction

Experimental Section
Chemicals and Reagents
Instrumentation
Synthesis
Results and Discussion
Conclusions
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References
- Gupta, V.K.; Jain, A.K.; Singh, L.P. Upendra Khurana Porphyrins as carrier in PVC based membrane potentiometric sensors for Nickel (II). Anal. Chim. Acta 1997, 355, 33–41. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, A.K.; Singh, L.P. Upendra Khurana, Pankaj Kumar Molybdate Sensor based on 5,10,15,20-tetraphenylporphyrinatocobalt Complex in PVC matrix. Anal. Chim. Acta 1999, 379, 201–208. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kumar, A.; Mangla, R. Protoporphyrin IX dimethyl ester as active material in PVC matrix.membranes for the fabrication of Zinc (II) selective sensor. Sens. Actuators B 2001, 76, 617–623. [Google Scholar] [CrossRef]
- Prasad, R.; Gupta, V.K.; Kumar, A. Metallo-tetraazaporphyrin based anion sensors: Regulation of sensor characteristics through central metal ion coordination. Anal. Chim. Acta 2004, 508, 61–70. [Google Scholar] [CrossRef]
- Gupta, V.K.; Prasad, R.; Kumar, A. Magnessium-tetrazaporphyrin incorporated PVC matrix as a new material for fabrication of Mg2+ selective potentiometric sensor. Talanta 2004, 63, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Tidwell, C.P.; Alexander, L.A.; Fondren, L.D.; Belmore, K.; Nikles, D.E. Synthesis and characterization of 5,10,15,20-tetra(N-ethyl-3-carbazolyl) porphyrin. Indian J. Chem. Sect. B 2007, 46B, 1658–1665. [Google Scholar]
- Wolberg, A.; Manassen, J. Electrochemical and electron paramagnetic resonance studies of metalloporphyrins and their electrochemical oxidation products. J. Am. Chem. Soc. 1970, 92, 2982–2991. [Google Scholar] [CrossRef] [PubMed]
- Kadish, K.M. The Electrochemistry of Metalloporphyrins in Nonaqueous Media in Progress in Inorganic Chemistry; John Wiley: New York, NY, USA, 1986; pp. 435–605. [Google Scholar]
- Felton, R.H.; Lintschitz, H. Polarographic Reduction of Porphyrins and Electron Spin Resonance of Porphyrin Anions. J. Am. Chem. Soc. 1966, 88, 1113–1116. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tidwell, C.P.; Fondren, L.D.; Nikles, D.E. Copper 5,10,15,20-Tetra(N-ethyl-3-carbazolyl) Porphyrin. Molbank 2011, 2011, M716. https://doi.org/10.3390/M716
Tidwell CP, Fondren LD, Nikles DE. Copper 5,10,15,20-Tetra(N-ethyl-3-carbazolyl) Porphyrin. Molbank. 2011; 2011(1):M716. https://doi.org/10.3390/M716
Chicago/Turabian StyleTidwell, Cynthia P., L. Dalila Fondren, and David E. Nikles. 2011. "Copper 5,10,15,20-Tetra(N-ethyl-3-carbazolyl) Porphyrin" Molbank 2011, no. 1: M716. https://doi.org/10.3390/M716
APA StyleTidwell, C. P., Fondren, L. D., & Nikles, D. E. (2011). Copper 5,10,15,20-Tetra(N-ethyl-3-carbazolyl) Porphyrin. Molbank, 2011(1), M716. https://doi.org/10.3390/M716