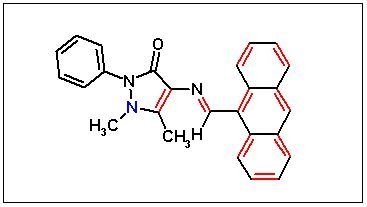
4-[(Anthracen-9-ylmethylene)amino]-1,5-dimethyl-2-phenyl-1,2-dihydropyrazol-3-one
Abstract
:Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References
- Brzozowski, Z.; Czewski, F.S.; Gdaniec, M. Synthesis, structural characterization and antitumor activity of novel 2,4-diamino-1,3,5-triazine derivatives. Eur. J. Med. Chem. 2000, 35, 1053–1064. [Google Scholar] [CrossRef]
- Husain, K.; Abid, M.; Azam, A. Novel Pd(II) complexes of 1-N-substituted 3-phenyl-2-pyrazoline derivatives and evaluation of antiamoebic activity. Eur. J. Med. Chem. 2008, 43, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.; Yusuf, M.; Khan, S.A.; Sahota, P.P.; Pandove, G. Synthesis, studies and invitro-antibacterial activity of N- substituted-5-(furan-2-yl)-phenyl pyrazolines. Arabian J. Chem. 2011, in press. [Google Scholar]
- Amir, M.; Kumar, H.; Khan, S.A. Synthesis and pharmacological evaluation of pyrazoline derivatives as new anti-inflammatory and analgesic agents. Bioorg. Med. Chem. Lett. 2008, 18, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Menozzi, G.; Mosti, L.; Merello, L.; Piana, A.; Armani, U.; Ghia, M.; Angiola, M.; Mattioli, F. 4-Dialkylamino-1-(5-substituted or unsubstituted 1-phenyl-1H-pyrazol-4-yl)butan-1-ols: synthesis and evaluation of analgesic, anti-inflammatory and platelet anti-aggregating activities. Il Farmaco 2000, 55, 219–226. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Abdellatif, K.R.A.; Dong, Y.; Knaus, E.E. Synthesis of new 4-[2-(4-methyl(amino)sulfonylphenyl)-5-trifluoromethyl-2H-pyrazol-3-yl]-1,2,3,6-tetrahydropyridines: A search for novel nitric oxide donor anti-inflammatory agents. Bioorg. Med. Chem. 2008, 16, 8882–8888. [Google Scholar] [CrossRef] [PubMed]
- Banday, A.H.; Mir, B.P.; Lone, I.H.L.; Suri, K.A.; Kumar, H.M.S. Studies on novel D-ring substituted steroidal pyrazolines as potential anticancer agents. Steroids 2010, 75, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Asiri, A.M.; Khan, S.A. Synthesis and anti-bacterial activities of some novel schiff bases derived from aminophenazone. Molecules 2010, 15, 6850–6858. [Google Scholar] [CrossRef] [PubMed]

© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Asiri, A.M.; Khan, S.A. 4-[(Anthracen-9-ylmethylene)amino]-1,5-dimethyl-2-phenyl-1,2-dihydropyrazol-3-one. Molbank 2011, 2011, M725. https://doi.org/10.3390/M725
Asiri AM, Khan SA. 4-[(Anthracen-9-ylmethylene)amino]-1,5-dimethyl-2-phenyl-1,2-dihydropyrazol-3-one. Molbank. 2011; 2011(2):M725. https://doi.org/10.3390/M725
Chicago/Turabian StyleAsiri, Abdullah M., and Salman A. Khan. 2011. "4-[(Anthracen-9-ylmethylene)amino]-1,5-dimethyl-2-phenyl-1,2-dihydropyrazol-3-one" Molbank 2011, no. 2: M725. https://doi.org/10.3390/M725
APA StyleAsiri, A. M., & Khan, S. A. (2011). 4-[(Anthracen-9-ylmethylene)amino]-1,5-dimethyl-2-phenyl-1,2-dihydropyrazol-3-one. Molbank, 2011(2), M725. https://doi.org/10.3390/M725