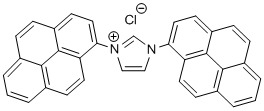
1,3-Bis(pyren-1-yl)imidazolium chloride (IPyr·HCl)
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgements
References
- Arduengo, A.J.; Harlow, R.L.; Kline, M. A Stable Crystalline Carbene. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Elison, M.; Fischer, J.; Köcher, C.; Artus, G.R.J. Metal complexes of N‑heterocyclic carbenes - a new structural principle for catalysts in homogeneous catalysis. Angew. Chem. Int. Ed. 1995, 34, 2371–2374. [Google Scholar] [CrossRef]
- Bantreil, X.; Broggi, J.; Nolan, S.P. N-Heterocyclic carbene containing complexes in catalysis. Annu. Rep. Prog. Chem. Sect. B 2009, 105, 232–263. [Google Scholar] [CrossRef]
- Scholl, M.; Ding, S.; Lee, C.W.; Grubbs, R.H. Synthesis and Activity of a New Generation of Ruthenium–Based Olefin Metathesis Catalysts Coordinated with 1,3–Dimesityl–4,5–dihydroimidazol–2–ylidene Ligands. Org. Lett. 1999, 1, 953–956. [Google Scholar] [PubMed]
- Enders, D.; Niemeier, O.; Henseler, A. Organocatalysis by N–Heterocyclic Carbenes. Chem. Rev. 2007, 107, 5606–5655. [Google Scholar] [CrossRef] [PubMed]
- Hintermann, L. Expedient syntheses of the N–heterocyclic carbene precursor imidazolium salts IPr·HCl, IMes·HCl and IXy·HCl. Beilstein J. Org. Chem. 2007, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Arduengo, A.J., III. Preparation of 1,3-disubstituted imidazolium salts. U.S. Patent 5,077,414, 31 December 1991. [Google Scholar]
- Imperato, G.; König, B. Acceleration of Suzuki–Miyaura– and Stille–type Coupling Reactions by Irradiation with Near–UV–A–Light. ChemSusChem 2008, 1, 993–996. [Google Scholar] [CrossRef] [PubMed]

© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Schroll, P.; König, B. 1,3-Bis(pyren-1-yl)imidazolium chloride (IPyr·HCl). Molbank 2011, 2011, M729. https://doi.org/10.3390/M729
Schroll P, König B. 1,3-Bis(pyren-1-yl)imidazolium chloride (IPyr·HCl). Molbank. 2011; 2011(2):M729. https://doi.org/10.3390/M729
Chicago/Turabian StyleSchroll, Peter, and Burkhard König. 2011. "1,3-Bis(pyren-1-yl)imidazolium chloride (IPyr·HCl)" Molbank 2011, no. 2: M729. https://doi.org/10.3390/M729
APA StyleSchroll, P., & König, B. (2011). 1,3-Bis(pyren-1-yl)imidazolium chloride (IPyr·HCl). Molbank, 2011(2), M729. https://doi.org/10.3390/M729