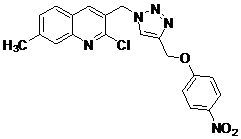
2-Chloro-7-methyl-3-({4-[(4-nitrophenoxy)methyl]-1H-1,2,3-triazol-1-yl}methyl)quinoline
Abstract
:Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgements
References
- O’Meara, S.M.; Cullum, N.A.; Majid, M.; Sheldon, T.A. Systematic review of antimicrobial agents used for chronic wounds. Br. J. Surg. 2001, 88, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Abdulqader, A.A.; Abdelkader, M.A.; Lien, E.J. Design, Synthesis, and pharmacological activities of 2-substituted 4-phenylquinolines as potential antidepressant drugs. J. Med. Chem. 1985, 28, 1394–1398. [Google Scholar]
- Kumar, S.; Bawa, S.; Drabu, S.; Kumar, R.; Panda, B.P. New 2-chloro-7-methylquinoline amine analogues as possible antimycotic agents. Lat. Am. J. Pharm. 2010, 29, 968–975. [Google Scholar]
- Kumar, S.; Bawa, S.; Gupta, H. Biological activities of quinoline derivatives. Mini Rev. Med. Chem. 2009, 14, 1648–1654. [Google Scholar] [CrossRef]
- Kumar, S.; Bawa, S.; Drabu, S.; Gupta, H.; Machwal, L.; Kumar, R. Synthesis, antidepressant and antifungal evaluation of novel 2-chloro-8-methylquinoline amine derivatives. Eur. J. Med. Chem. 2011, 46, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Bindu, P.J.; Mahadevan, K.M.; Satyanarayan, N.D.; Ravikumar Naik, T.R. Synthesis and DNA cleavage studies of novel quinoline oxime esters. Bioorg. Med. Chem. Lett. 2012, 22, 898–900. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Rizvi, S.U.F.; Siddiqui, H.L.; Ahmad, S.; Parvez, M.; Suliman, R. Antioxidant and antimicrobial studies of novel N′-(substituted-2-chloroquinolin-3-yl)methylidene-4-hydroxy-2H-1,2-benzothiazine-3-carbohydrazides-1,1-dioxides. Med. Chem. Res. 2012, 21, 2340–2348. [Google Scholar] [CrossRef]
- Shafran, E.A.; Bakulev, V.A.; Rozin, Y.A.; Shafran, Y.M. Condensed 1,2,3-triazoles (review). Chem. Heterocycl. Comp. 2008, 44, 1040–1069. [Google Scholar] [CrossRef]
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. 1,2,3-Triazole tethered β-lactam-chalcone bifunctional hybrids: Synthesis and anticancer evaluation. Eur. J. Med. Chem. 2012, 47, 594–600. [Google Scholar]
- Singh, P.; Raj, R.; Kumar, V.; Mahajan, M.P.; Bedi, P.M.S.; Kaur, T.; Saxena, A.K. Click chemistry: 1,2,3-Triazoles as pharmacophores. Chem. Asian J. 2011, 6, 2696–2718. [Google Scholar]
- Reddy, L.V.; Nakka, M.; Alishetty, S.; Ghosh, S.; Helliwell, M.; Khagga, M.; Mukherjee, A.K.; Pal, S. Synthesis of novel quinoline analogues of nimesulide: An unusual observation. J. Heterocycl. Chem. 2011, 48, 555–562. [Google Scholar] [CrossRef]
- Reddy, L.V.; Nallapati, S.B.; Beevi, S.S.; Mangamoori, L.S.; Khagga, M.; Pal, S. A "green" synthesis of N-(quinoline-3-ylmethylene)benzohydrazide derivatives and their cytotoxicity activities. J. Braz. Chem. Soc. 2011, 22, 1742–1749. [Google Scholar] [CrossRef]
- Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V.V.; Noodleman, L.; Sharpless, K.B.; Fokin, V.V. Copper(I)-catalyzed synthesis of azoles. DFT study predicts unprecedented reactivity and intermediates. J. Am. Chem. Soc. 2005, 127, 210–216. [Google Scholar] [CrossRef] [PubMed]


© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Praveena, K.S.S.; Murthy, N.Y.S.; Pal, S. 2-Chloro-7-methyl-3-({4-[(4-nitrophenoxy)methyl]-1H-1,2,3-triazol-1-yl}methyl)quinoline. Molbank 2013, 2013, M797. https://doi.org/10.3390/M797
Praveena KSS, Murthy NYS, Pal S. 2-Chloro-7-methyl-3-({4-[(4-nitrophenoxy)methyl]-1H-1,2,3-triazol-1-yl}methyl)quinoline. Molbank. 2013; 2013(2):M797. https://doi.org/10.3390/M797
Chicago/Turabian StylePraveena, Koduru Sri Shanthi, Nandula Yadagiri Sreenivasa Murthy, and Sarbani Pal. 2013. "2-Chloro-7-methyl-3-({4-[(4-nitrophenoxy)methyl]-1H-1,2,3-triazol-1-yl}methyl)quinoline" Molbank 2013, no. 2: M797. https://doi.org/10.3390/M797
APA StylePraveena, K. S. S., Murthy, N. Y. S., & Pal, S. (2013). 2-Chloro-7-methyl-3-({4-[(4-nitrophenoxy)methyl]-1H-1,2,3-triazol-1-yl}methyl)quinoline. Molbank, 2013(2), M797. https://doi.org/10.3390/M797