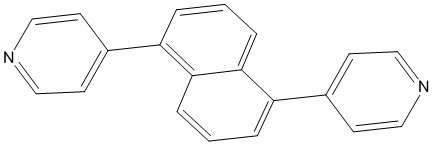
1,5-(4,4'-Dipyridyl)naphthalene
Abstract
:Introduction
Results and Discussion
Experimental Section
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References
- Culp, J.T.; Madden, C.; Kauffman, K.; Shi, F.; Matranga, C. Screening Hofmann compounds as CO2 sorbents: Non-traditional synthetic route to over 40 different pore-functionalized and flexible pillared cyanonickelates. Inorg. Chem. 2013, 52, 4206–4216. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-J.; Jouaiti, A.; Kyritsakas, N.; Hosseini, M.W. Molecular tectonics: Control of interpenetration in cuboid 3-D coordination networks. CrystEngComm 2011, 13, 776–778. [Google Scholar] [CrossRef]
- Hauptvogel, I.M.; Bon, V.; Grünker, R.; Baburin, I.A.; Senkovska, I.; Mueller, U.; Kaskel, S. A family of 2D and 3D coordination polymers involving a trigonal tritopic linker. Dalton Trans. 2012, 41, 4172–4179. [Google Scholar] [CrossRef] [PubMed]
- Karagiaridi, O.; Bury, W.; Tylianakis, E.; Sarjeant, A.A.; Hupp, J.T.; Farha, O.K. Opening metal-organic frameworks vol. 2: Inserting longer pillars into pillared-paddlewheel structures through solvent-assisted linker exchange. Chem. Mater. 2013, 25, 3499–3503. [Google Scholar] [CrossRef]
- Wolf, C.; Ghebremariam, B.T. Synthesis of atropisomeric 1,8-bis(4′,4′-dipyridyl)naphthalenes from 4-trimethylstannylpyridines. Synthesis 2002, 2002, 749–752. [Google Scholar] [CrossRef]
- Mei, X.; Wolf, C. Conformational polymorphism of 1,8-dipyridylnaphthalene and encapsulation of chains of fused cyclic water pentamers in a hydrophobic crystal environment. CrystEngComm 2006, 8, 377–380. [Google Scholar] [CrossRef]
- Mei, X.; Liu, S.; Wolf, C. Template-controlled face-to-face stacking of olefinic and aromatic carboxylic acids in the solid state. Org. Lett. 2007, 9, 2729–2732. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.B.J.; Bučar, D.-K.; Sokolov, A.N.; Friščić, T.; Robinson, C.N.; Bilal, M.Y.; Sinada, N.G.; Chevannes, A.; MacGillivray, L.R. General application of mechanochemistry to templated solid-state reactivity: Rapid and solvent-free access to crystalline supermolecules. Chem. Commun. 2008, 5713–5715. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.B.J.; Sokolov, A.N.; Bučar, D.-K.; Mariappan, S.V.S.; Mwangi, M.T.; Tiedman, M.C.; MacGillivray, L.R. Applications of hydrogen-bond-acceptor templates to direct ‘in-phase’ reactivity of a diene diacid in the solid state. Photochem. Photobiol. Sci. 2011, 10, 1384–1386. [Google Scholar] [CrossRef] [PubMed]
- Beyler, M.; Heitz, V.; Sauvage, J.-P. The dual role of Cu(I) as a protective group and a template in the synthesis of a tetra-rhodium(III)porphyrin [2]catenane. New J. Chem. 2010, 34, 1825–1829. [Google Scholar] [CrossRef]
- Beyler, M.; Heitz, V.; Sauvage, J.-P. Coordination chemistry-assembled porphyrinic catenanes. J. Am. Chem. Soc. 2010, 132, 4409–4417. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, H.H.; Whitehurst, J.S. 17. The tetrazotisation of 1:5- and 1:8-naphthylenediamines and the tetranitration of 1:5- and 1:8-di-p-toluenesulphonamidonaphthalenes. J. Chem. Soc. 1947, 80–81. [Google Scholar] [CrossRef]
- Biradha, K.; Hongo, Y.; Fujita, M. Open square-grid coordination polymers of the dimensions 20 × 20 Å: Remarkably stable and crystalline solids even after guest removal. Angew. Chem. Int. Ed. 2000, 39, 3843–3845. [Google Scholar] [CrossRef]
- Marin, G.; Andruh, M.; Madalan, A.M.; Blake, A.J.; Wilson, C.; Champness, N.R.; Schröder, M. Structural diversity in metal-organic frameworks derived from binuclear alkoxo-bridged copper(II) nodes and pyridyl linkers. Cryst. Growth Des. 2008, 8, 964–975. [Google Scholar] [CrossRef]



© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, M.R.; Boeré, R.T. 1,5-(4,4'-Dipyridyl)naphthalene. Molbank 2015, 2015, M845. https://doi.org/10.3390/M845
Hassan MR, Boeré RT. 1,5-(4,4'-Dipyridyl)naphthalene. Molbank. 2015; 2015(1):M845. https://doi.org/10.3390/M845
Chicago/Turabian StyleHassan, Mohammad R., and René T. Boeré. 2015. "1,5-(4,4'-Dipyridyl)naphthalene" Molbank 2015, no. 1: M845. https://doi.org/10.3390/M845
APA StyleHassan, M. R., & Boeré, R. T. (2015). 1,5-(4,4'-Dipyridyl)naphthalene. Molbank, 2015(1), M845. https://doi.org/10.3390/M845