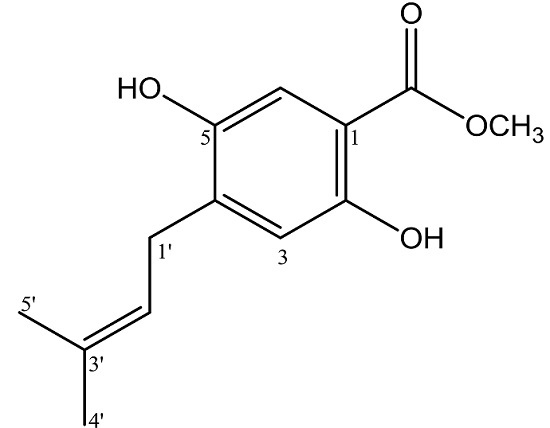
Methyl 2,5-Dihydroxy-4-(3′-methyl-2′-butenyl)benzoate
Abstract
:1. Introduction
2. Result and Discussion
3. Material and Methods
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation
3.4. DPPH Radical Scavenging
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ozawa, M.; Kawamata, S.; Etoh, T.; Hayashi, M.; Komiyama, K.; Kishida, A.; Kuroda, C.; Ohsaki, A. Structures of new erythrinan alkaloids and nitric oxide production inhibitors from Erythrina crista-galli. Chem. Pharm. Bull. 2010, 58, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Watjen, W.; Suckow, A.K.; Rohrig, R.; Kulawik, A.; Wright, C.W.; Passreitrer, C.M. Prenylated flavonoid derivatives from the bark of Erythrina addisoniae. J. Nat. Prod. 2008, 71, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Yenesew, A.; Midiwo, J.O.; Heydenreich, M.; Schanzenbach, D.; Peter, M.G. Two isoflavanones from the stem bark of Erythrina sacleuxii. Phytochemistry 2000, 55, 457–459. [Google Scholar] [CrossRef]
- Innok, P.; Rukachaisirikul, T.; Phongpaichit, S.; Suksamrarn, A. Fuscacarpans A-C, new pterocarpans from the stems of Erytrina fusca. Fitoterapia 2010, 81, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.; Le, T.V.T.; Thuong, P.T.; Dao, T.T.; Ndinteh, D.T.; Mbafor, J.T.; Kang, K.W.; Oh, W.K. Cytotoxic and PTP1B inhibitory activities from Erythrina abyssinica. Bioorg. Med. Chem. Lett. 2009, 18, 6745–6749. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.; Na, M.K.; Dao, T.T.; Ndinteh, D.T.; Mbafor, J.T.; Park, J.; Cheong, H.; Oh, W.K. New stilbenoid with inhibitory activity on viral neuraminidases from Erythrina addisoniae. Bioorg. Med. Chem. Lett. 2010, 20, 6430–6434. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Gorzalczany, S.; Martino, V.; Acevedo, C.; Sterner, O.; Anke, T. Metabolites from endophytes of the medicinal plant Erytrina crista-galli. Z. Naturforsch. C 2005, 60, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, P.; Crombie, L.; Harper, M.F.; Rossiter, J.T.; Sanders, M.; Whiting, D.A. Mechanism and stereochemistry of the enzyme-catalysed formation of a 2,2-dimethylchromene ring from a prenylated phenol: conversion of rot-2′-enonic acid into deguelin by deguelin cyclase. J. Chem. Soc. Perkin Trans 1 1992, 13, 1685–1697. [Google Scholar] [CrossRef]
- Tanjung, M.; Tjahjandarie, T.S.; Sentosa, M.H. Antioxidant and cytotoxic agent from the rhizomes of Kaempferia pandurata. Asian Pac. J. Tropical Dis. 2013, 3, 401–404. [Google Scholar] [CrossRef]
- Tanjung, M.; Saputri, R.D.; Tjahjandarie, T.S. Antioxidant activity of two isomeric benzoxepin derivatives from the stem bark of Bauhinia acuelata L. J. Chem. Pharm. Res. 2014, 6, 705–708. [Google Scholar]


| No. C | δH ppm (mult, J Hz) | δC ppm | HMBC |
|---|---|---|---|
| 1 | - | 105.4 | - |
| 2 | - | 162.1 | - |
| 3 | 6.39 (s, 1H) | 103.4 | C-1, C-2, C-4, C-5 |
| 4 | - | 119.2 | - |
| 5 | - | 160.9 | - |
| 6 | 7.56 (s, 1H) | 131.1 | C-2, C-4, C=O |
| 1′ | 3.27 (d, 7.0, 2H) | 28.9 | C-3, C-4, C-5, C-2′, C-3′ |
| 2′ | 5.28 (tm, 7.5, 1H) | 121.6 | C-4′, C-5′ |
| 3′ | - | 135.0 | - |
| 4′ | 1.77 (s, 3H) | 25.8 | C-2′, C-3′, C-5′ |
| 5′ | 1.76 (s, 3H) | 17.9 | C-2′, C-3′, C-4′ |
| C=O | - | 170.4 | - |
| 2-OH | 10.79 (s, 1H) | - | C-1, C-2, C-3 |
| OCH3 | 3.91 (s, 3H) | 52.0 | C=O |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tjahjandarie, T.S.; Saputri, R.D.; Tanjung, M. Methyl 2,5-Dihydroxy-4-(3′-methyl-2′-butenyl)benzoate. Molbank 2016, 2016, M892. https://doi.org/10.3390/M892
Tjahjandarie TS, Saputri RD, Tanjung M. Methyl 2,5-Dihydroxy-4-(3′-methyl-2′-butenyl)benzoate. Molbank. 2016; 2016(2):M892. https://doi.org/10.3390/M892
Chicago/Turabian StyleTjahjandarie, Tjitjik Srie, Ratih Dewi Saputri, and Mulyadi Tanjung. 2016. "Methyl 2,5-Dihydroxy-4-(3′-methyl-2′-butenyl)benzoate" Molbank 2016, no. 2: M892. https://doi.org/10.3390/M892
APA StyleTjahjandarie, T. S., Saputri, R. D., & Tanjung, M. (2016). Methyl 2,5-Dihydroxy-4-(3′-methyl-2′-butenyl)benzoate. Molbank, 2016(2), M892. https://doi.org/10.3390/M892