3,5-Dimethoxy-2-[(4-methoxyphenyl)diazenyl]phenol
Abstract
:1. Introduction
2. Results
3. Materials and Methods
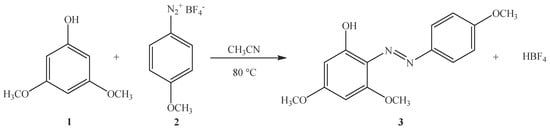
Synthesis of 3,5-Dimethoxy-2-[(4-methoxyphenyl)diazenyl]phenol (3)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zollinger, H. Color Chemistry, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Broadbent, A.D. Basic Principles of Textile Coloration, 1st ed.; Society of Dyers and Colourists: Bradford, UK, 2001. [Google Scholar]
- Zollinger, H. Diazo Chemistry I, 1st ed.; VCH: Weinheim, Germany, 1994. [Google Scholar]
- Chehimi, M. Aryl Diazonium Salts; Wiley-VCH Verlag and Co.: Weinheim, Germany, 2012. [Google Scholar]
- Boga, C.; Cino, S.; Micheletti, G.; Padovan, D.; Prati, L.; Mazzanti, A.; Zanna, N. New azo-decorated N-pyrrolidinylthiazoles: Synthesis, properties and an unexpected remote substituent effect transmission. Org. Biomol. Chem. 2016, 14, 7061–7068. [Google Scholar] [CrossRef] [PubMed]
- Boga, C.; Micheletti, G.; Cino, S.; Fazzini, S.; Forlani, L.; Zanna, N.; Spinelli, D. C–C coupling between trinitrothiophenes and triaminobenzenes: Zwitterionic intermediates and new all-conjugated structures. Org. Biomol. Chem. 2016, 14, 4267–4275. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, E.; Boga, C.; Forlani, L.; Tozzi, S.; Micheletti, G.; Cino, S. Ring Closure of Azo Compounds to 1,2-Annulated Benzimidazole Derivatives and Further Evidence of Reversibility of the Azo- Coupling Reaction. J. Org. Chem. 2015, 80, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- Micheletti, G.; Boga, C.; Pafundi, M.; Pollicino, S.; Zanna, N. New electron-donor and -acceptor architectures from benzofurazans and sym-triaminobenzenes: Intermediates, products and an unusual nitro group shift. Org. Biomol. Chem. 2016, 14, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Arzhantsev, S.Y.; Tahra, T. Femtosecond Time-Resolved Fluorescence Study of Photoisomerization of trans-Azobenzene. J. Phys. Chem. A 2001, 105, 8123–8129. [Google Scholar] [CrossRef]
- Yoshino, J.; Kano, N.; Kawashima, T. Synthesis of the most intensely fluorescent azobenzene by utilizing the B–N interaction. Chem. Commun. 2007, 6, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Kano, N.; Furuta, A.; Kambe, T.; Yoshino, J.; Shibata, Y.; Kawashima, T.; Mizorogi, N.; Nagase, S. 2,2′-Diborylazobenzenes with Double N–B Coordination: Control of Fluorescent Properties by Substituents and Redox Reactions. Eur. J. Inorg. Chem. 2012, 2012, 1584–1587. [Google Scholar] [CrossRef]
- Hansen, M.J.; Lerch, M.M.; Szymanski, W.; Feringa, B.L. Direct and Versatile Synthesis of Red-Shifted Azobenzenes. Angew. Chem. Int. Ed. 2016, 55, 13514–13518. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micheletti, G.; Telese, D.; Boga, C. 3,5-Dimethoxy-2-[(4-methoxyphenyl)diazenyl]phenol. Molbank 2020, 2020, M1152. https://doi.org/10.3390/M1152
Micheletti G, Telese D, Boga C. 3,5-Dimethoxy-2-[(4-methoxyphenyl)diazenyl]phenol. Molbank. 2020; 2020(3):M1152. https://doi.org/10.3390/M1152
Chicago/Turabian StyleMicheletti, Gabriele, Dario Telese, and Carla Boga. 2020. "3,5-Dimethoxy-2-[(4-methoxyphenyl)diazenyl]phenol" Molbank 2020, no. 3: M1152. https://doi.org/10.3390/M1152
APA StyleMicheletti, G., Telese, D., & Boga, C. (2020). 3,5-Dimethoxy-2-[(4-methoxyphenyl)diazenyl]phenol. Molbank, 2020(3), M1152. https://doi.org/10.3390/M1152